Journal of APPLIED BIOMEDICINE
ISSN 1214-0287 (on-line)
ISSN 1214-021X (printed)
Volume 9 (2011), No 2, p 111-118
DOI 10.2478/v10136-009-0036-2
Ectopic osteogenesis with immortalized human bone marrow stromal stem cells and heterologous bone
Yong Teng, Yunyu Hu, Xusheng Li, Yucheng Guan, Junhao Gui
Address: Yong Teng, Department of Orthopedics, Orthopedic Center of PLA, Urumqi General Hospital, Lanzhou Military Region, Xinjiang Uygur
Autonomous Region 830000, China
tangyunn@yeah.net
Received 18th October 2010.
Published online 2nd November 2010.
Full text article (pdf)
Abstract in xml format
Summary
Key words
Introduction
Materials and methods
Results
Discussion
Conclusion
References
SUMMARY
To resolve the problem of the insufficient availability of seed cells and to provide seed cells for tissue engineering research, an immortalized
human bone marrow stromal stem cell line (MSCxj cells) was established in our department to investigate the ectopic osteogenesis of MSCxj
cells.
MSCxjs were grown with a heterogeneous bone scaffold for 48 h. Three groups were included: A: MSCxjs of 35 PDs were maintained with heterogeneous
bone; B: MSCxjs of 128 PDs were maintained with heterogeneous bone; and C: heterogeneous bone alone. Tetracycline fluorescence staining, H&E;
staining, and ponceau staining, immunohistochemistry and bone histomorphometry were performed. At the same time, scanning electron microscopy was
conducted to detect the growth of MSCxjs and heterogeneous bone.
Scanning electron microscopy showed favorable adherence of MSCxjs to heterogeneous bone. A large number of newly generated filamentous
extracellular matrix and fine granular materials were found to cover the cells. The results from staining showed that the osteogenesis was not
obvious in group A/B 4 weeks after transplantation. Eight weeks after implantation, osteoid matrix deposition was noted in and around the
heterogeneous bone in group A/B. Twelve weeks after implantation, osteogenesis was increased in group A/B. There were no significant differences
in the time course for bone formation and the amount of newly generated bone between group A/B.
Like primary hBMSCs, MSCxj cells have favourable ectopic osteogenesis and can be applied as seeded cells in bone tissue engineering.
KEY WORDS
tissue engineering; immortalization; human; bone marrow stromal cells; ectopic osteogenesis; xenografts
INTRODUCTION
Numerous factors, including trauma, infection and
cancer can cause focal or segmental bone loss and
generate large gaps between bones, also known as
bone defects. A majority of bone defects are difficult
to spontaneously heal completely, and bone nonunion
is frequently observed in clinical practice. A number
of researchers have conducted studies to investigate
bone defects (Zhao et al. 1998, Bauer and Muschler
2000, Byun et al. 2010, Hesse et al. 2010). Recently,
as the relevant technology has developed, the repair of
bone defects has more often been carried out through
tissue engineering. Tissue engineering bone
constructed by scaffold, seed cells and growth factors
provides a promising strategy for the treatment of
bone defects.
Human telomerase reverse transcriptase (hTERT)
was introduced into the human bone marrow stromal
stem cell line (MSCxj) through liposomes to establish
immortalized MSCxjs. The biological features of
MSCxjs and functions of adult stem cells have been
determined. These MSCxjs have biological features
similar to normal human bone marrow stromal stem
cells and the plasticity of adult stem cells. MSCxjs
can differentiate into osteoblasts in vitro (Teng et al.
2007a, b). In the present study, the in vitro ectopic
osteogensis of MSCxjs was further investigated and
the prospect of using MSCxjs in bone tissue
engineering was explored.
MATERIALS AND METHODS
Main reagents and materials
The following were used in our study: mouse
anti-human osteocalcin monoclonal antibodies (R&D;,
USA), tetracycline (Sigma, USA), a SM2500E hard
tissue microtome and an MPS60 image acquisition
and analysis system (Leica, Germany). The reagents
used for cell culture were as follows: L-DMEM-low
glucose, trypsin (Gibco BRL, USA), fetus bovine
serum (FBS; Hyclone, USA), dexamethasone,
beta-glycerol phosphate, vitamin C (Sigma, USA),
Percoll separating solution (Pharmacia, USA) a
24-well plate, a 50 ml flask (Coster, USA), Methyl
thiazolyl tetrazolium (MTT; Sino-American
Biotechnology Co., Ltd., China) and a CO2 incubator
(Heraeus, Germany). The complete medium was
L-DMEM containing 10% FBS, 100 U/ml penicillin
and streptomycin. The conditional medium for
osteogenesis was the complete medium supplemented
with 10-8 mol/l dexamethasone, 50 mg/l beta-glycerol
phosphate, and 10mmol/l vitamin C. The
immortalized human bone marrow stromal stem cells
were initially prepared by the Orthopaedic Institute of
the Fourth Military Medical University and named
MSCxj. Antigen-free bovine cancellous bone was
developed as the scaffold by the Orthopaedic Institute
of the Fourth Military Medical University.
Grouping
Eighteen healthy female nude mice (4-6 weeks)
weighing 12-20 g were purchased from the Animal
Center of the Forth Military Medical University and
were treated as follows: group A: heterologous bone
plus MSCxj (35PDs) (n=12); group B: heterologous
bone plus MSCxj (128PDs) (n=12); group C:
heterologous bone alone (n=12).
Culture of MSCxj
The immortalized MSCxj cells were prepared by the
Orthopaedic Institute of the Fourth Military Medical
University and labelled. The thawed out MSCxj cells
from passage 35 and passage 128 were maintained in
an L-DMEM containing 10% serum. Cell adherence
and growth were observed under a phase contrast
microscope. When the cell confluence reached 90%
(after about 5 days of culture), these cells were
digested with 0.25% trypsin (1:3 v/v) followed by
culture. The cryopreserved cells were thawed and
maintained in the medium until 35 population
doublings or 128 PDs. When the cell number
reached 2x109, theses cells were digested and then
mixed with phosphate-buffered saline (PBS; 5 ml)
followed by centrifugation at 250 g for 10 min. These
cells were washed with PBS again and the cells were
harvested followed by preparation of cell suspension
(2x109/ml) with 4.5 ml of L-DMEM containing 10%
serum.
Preparation of heterologous bones
A bovine heterologous bone scaffold was developed
by the Orthopaedic Institute of the Fourth Military
Medical University. The degreased and deproteined
bovine bone was cut into 3x3x3 mm3 cancellous
bones and washed with double distilled water. These
cancellous bones were dried at room temperature for
24 h, wrapped with aluminum foil and then sterilized
by cobalt 60 radiation. They were then immersed in
PBS for 24 h before use.
Integration of MSCxj and scaffold
The cells were divided into three groups. In group A
and B, MSCxj cells of 35 PDs and 128 PDs,
respectively, were maintained with the bovine
cancellous bone (n=12 per group) when the cell
number reached 2x109. Group C included the bovine
cancellous bone alone (n=12). Under aseptic
conditions, the disinfected bovine cancellous bones
from the different groups were put into 3 wells
(8-well plate) independently. In group A and group
B, the MSCxj cell suspension was maintained with
bovine cancellous bones through repeated
precipitation. Briefly, the cell suspension was
dropped onto the pre-moistened bone scaffolds which
were then incubated in an incubator. This procedure
was repeated once every 4 h for a total of 48 h. In
group C, the cell suspension was replaced with PBS.
Forty eight hours later, the cells completely adhered
to the scaffolds and cell growth was favourable as
demonstrated by the scanning electron microscopy.
These scaffolds were then grown in the L-DMEM
osteogenic medium (containing 10% FBS, 100 U/ml
penicillin and streptomycin, 1x10-8 mol/l
dexamethasone, 50 mg/l beta glycerophosphate and
10 mmol/l vitamin C) for 18 d and the complex of
MSCxj cells and bone scaffolds was obtained for
further use.
Scanning electron microscopy (SEM) was
performed to detect the growth of cells on scaffolds
48 h and 18 d before implantation. The scaffolds were
washed with PBS twice and fixed in 3%
glutaraldehyde. Dehydration was performed with
acetonitrile in a series of concentrations. Critical
evaporation and metal spraying were conducted
followed by SEM.
Ectopic osteogensis in nude mice
The samples in group A, B and C were paired and a
total of 18 subgroups were established. The mice were
intraperitoneally anesthetized with 10 g/l sodium
pentobarbital (60 mg/kg), a 3 mm incision made at the
lateral back of each mouse (n=18) and the complex
was implanted. The wound was sutured, the animals
numbered and then maintained in cages.
The nude mice were sacrificed 4, 8, and 12 weeks
after implantation (6 mice per time point) and each
group (A, B and C) had 4 samples at each time point.
Ten days before sacrifice at week 4 and 8, animals
were fed with chow supplemented with tetracycline as
a fluorescence marker for observation of ectopic
osteogenesis. Osteoid was observed 8 weeks after
implantation. Ten days and 3 days before sacrifice at
week 12, animals were intraperitoneally injected with
tetracycline hydrochloride (25 mg/kg) for 3 consecutive days for fluorescent double labelling and
thereafter observation of ectopic osteogenesis. The
animals were killed through cervical dislocation.
Gross observation
The following were noted: contamination by bacteria
and fungi, survival and wound healing rate of the
mice following implantation, and the color of and
coverings on the scaffolds.
Histological examination
Tissues were fixed in 10% formaldehyde and
dehydrated with ethanol in a series of concentrations.
Transparentization was performed with toluene and
then tissues were immersed in methyl acrylic acid
methyl ester followed by embedding (Lv and Yang
1999, Lv et al. 2002, 2006). The tissues were cut
consecutively into 5 microm sections followed by
mounting and observation of tetracycline
fluorescence. In addition, H&E; staining and ponceau
S red staining and immunohistochemistry for
osteocalcin were carried out.
Morphometry
The tissues were consecutively cut into 5 m sections
(10 sections) at a low magnification (x10) and after
HE staining the levels of osteogenesis were
established. The areas with newly generated bones
and areas of implanted scaffold were determined with
an image analysis system and the proportion of areas
with newly generated bones was calculated as follow:
proportion (%) = (areas with newly generated bones/
areas of implanted scaffold) x100%.
Statistics
Data were expressed as means ± standard deviation
and analyzed with SPSS statistic software.
Comparisons between two groups were performed
with t test at the significance level 2alpha=0.05.
RESULTS
Features in three-dimensional culture
MSCxjs were maintained with heterologous bones
for 48 h, and cells were found on the surface and in
the pores of the cancellous bones. The cells were
spindle and adherent to the wall. Long cell processes
were noted. The cells interacted with each other over
time. A large amount of filamentous extracellular
matrix and small granular materials were found
before implantation and covered these cells (Fig. 1).
There was no contamination by bacterial or fungus
infection.
Observation of ectopic osteogenesis
Gross features
The complex of MSCxj/ heterologous bone was
implanted. The wound healing was acceptable and all
mice survived. Four weeks after implantation, fibrous
tissues covered the scaffolds and white heterologous
bones were frequently observed. Eight weeks after
implantation, scaffolds were covered with dark red
bone tissues and characterized by red and white bone
tissues. Twelve weeks after implantation, the
scaffolds were smooth and dark red. However, the
scaffolds in control group were white (Fig. 2).
H&E; staining, ponceau staining and tetracycline
fluorescence staining
Results from H&E; staining, ponceau staining and
tetracycline fluorescence staining showed that osteogenesis was not obvious in group A/B 4 weeks
after implantation. Eight weeks after implantation,
osteoid matrix deposition was noted around and in the
pores of heterogeneous bones in group A/B. In
addition, osteoblast-like cells rich in cytoplasm were
found and some osteogenesis was observed, but a
majority of pores were filled with fibrous tissue.
Twelve weeks after implantation, osteogenesis was
increased in group A/B. The newly generated bones
were located in the pores and nearly normal bone
marrow tissues were also found. In the cross section,
the scaffolds were surrounded by newly generated
bones, and lamellar bones formed. The progressive
growth of ectopic bones was noted by tetracycline
fluorescence staining. There were no significant
differences in the time course for bone formation and
the amount of newly generated bone between group
A/B. Osteogenesis was not observed in the control
group and only a small amount of fibrous tissues were
found on the ectopic bones (Fig. 3).
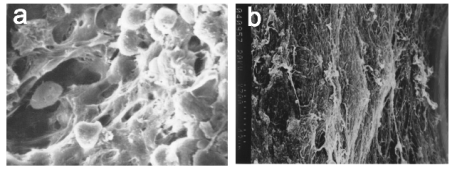
Fig. 1. Scanning electron microscopy for MSCxjs; a) MSCxjs were maintained with heterologous bones for 48 h (x1000);b) MSCxjs were
maintained with heterologous bones for 18 d. A large amount of filamentous extracellular matrix and small
granular materials were found before implantation (x600).
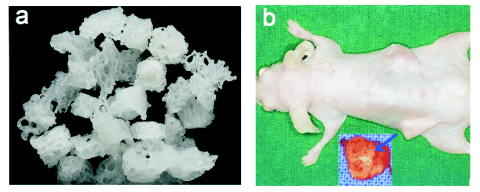
Fig. 2. Heterologous bones scaffolds and subcutaneous implantation; a) heterologous bones scaffolds; b) 12 weeks after the
complex of MSCxj/ heterologous bone was subcutaneously implanted in the nude mice. The blue arrow indicates the heterologous
bone.
There was no degradation of ectopic bones in
group A, B or C.
Immunohistochemistry for osteocalcin
Immunohistochemistry for osteocalcin showed
positive staining in the lacunae as shown by arrows
(Fig. 4).
Morphometry
Osteogenesis was not obvious in group C at all time
points and in groups A and B at 4 weeks after
implantation. Therefore, the difference in osteo-genesis was not compared between the three groups.
The proportion of newly generated bones in group A
and group B was 5.64%±2.68% and 4.92%±2.95%,
respectively, at 8 weeks after implantation and
13.94%±2.21% and 14.34%±3.46%, respectively, at
12 weeks after implantation. Statistical analysis did
not show a significant difference between group A
and group B at the same time point (statistically significant). Furthermore, the proportions of newly
generated bones in both group A and group B at
12 weeks after implantation were markedly higher
than those at 8 weeks after implantation (statistically
significant).
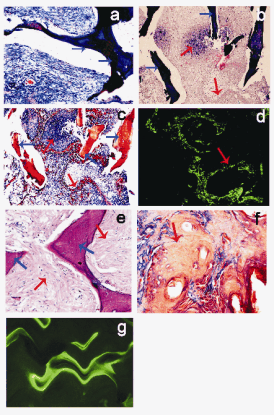
Fig. 3. Features of ectopic osteogenesis: H&E; staining,
ponceau staining and tetracycline fluorescence staining;
a) heterologous bone alone (ponceau staining, group C,
x100); b) 8 weeks after implantation of
MSCxj/heterologous bone in nude mice (H&E; staining,
group A, x100); c) 8 weeks after implantation of
MSCxj/heterologous bone (ponceau staining, group A,
x100); d) 8 weeks after implantation of
MSCxj/heterologous bone (tetracycline fluorescence
staining, group A, x100); e) 12 weeks after implantation of
MSCxj/heterologous bone ( H&E; staining, group B, x400);
f) 12 weeks after implantation of MSCxj/heterologous bone
(ponceau staining, group B, x400); g) 12 weeks after
implantation of MSCxj/heterologous bone (tetracycline
fluorescence staining, group B, x400). The blue arrow
indicates the heterologous bone; the red arrow indicates the
ectopic osteogenesis.
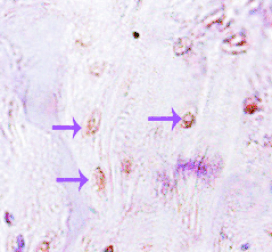
Fig. 4. Immunohistochemistry for osteocalcin 12 weeks
after the complex of MSCxj/heterologous bone was
subcutaneously implanted in nude mice (group B, x200).
The purple arrow indicates the osteocytes.
DISCUSSION
MSCxj act as seed cells in bone tissue engineering, a
discipline where seed cells are a key and basic factor.
The ideal seed cells should have the following
characteristics (Hodgkinson et al. 2009, Cordonnier
et al. 2010): (1) It is easy to obtain these seed cells
with minimal injury; (2) Seed cells should have the
characteristic of directed differentiation into
osteoblasts; (3) Seed cells have potent reproductive
capacity and an appropriate amount of cells can be
obtained. Bone marrow stromal stem cells (BMSCs)
have been widely applied in bone tissue engineering
in the past decade. However, cell senescence after
repeated passaging, the difficulty of collecting a
sufficient number of in a timely way and lack of
standard cell lines in the laboratories are the key
issues in the application of BMSCs (Hodgkinson et
al. 2009, Cordonnier et al. 2010, Richardson et al.
2010).
The problem of cell senescence can be resolved
through immortalization of bone marrow stromal stem
cells (MSCxjs), and the characteristics of adult stem
cells of BMSCs are then preserved. In the present
study, after osteogenic induction, MSCxjs and ectopic
bones were implanted in the nude mice and the
ectopic osteogenesis was observed. The results
showed that osteogenesis was active and the newly
generated bones had nearly normal bone structure.
But osteogenesis was not observed in the mice treated
with MSCxjs alone. Immunohistochemistry showed
osteocalcin expression in osteoblasts or osteocytes.
Our results indicated that MSCxjs cells have the
characteristic of ectopic osteogenesis which is similar
to primary BMSCs and can be applied as seed cells in
the experiments of bone tissue engineering. In
addition, a sufficient number of MSCxjs cells can be
obtained and these cells may become a standard cell
line for research in the laboratories.
The other problem in the application of BMSCs in
bone tissue engeering as seed cells is the difficulty of
timely collection; the collection of BMSCs from bone
marrow puncture to harvesting in sufficient quantities
is time consuming and liable to failure (Hodgkinson
et al. 2009, Zou et al. 2009, Jukes et al. 2010).
MSCxjs can be applied to establish a cell bank which
solves this problem, but the transplant rejection of
allogeneic cells should be further studied. Some
researchers speculate that BMSCs have extremely
weak immunogenicity; others even propose that these
cells have no immunogenicity, and therefore,
allogeneic bone marrow stromal stem cell
transplantation is feasible, and favourable results can
achieved in animal studies (Itescu et al. 2003,
Poncelet et al. 2007, Ren et al. 2010). However, some
researchers have an opposite opinion. They postulate
that BMSCs still have immunogenicity and results
from animal studies cannot be applied to humans. The
higher order the animals, the more obvious is the
immunological rejection (Spees et al. 2004, Nauta et
al. 2006). Therefore, the immunological rejection of
MSCxjs, a type of seed cells in bone tissue
engineering, should be further clarified. Because of
the possibility of immunological rejection,
implantation of these cells has been performed in the
present study only in nude mice with
immunodefficiency. The subcutaneous blood supply
in nude mice is not rich and therefore, the osteogenic
ability of these cells in unit time is not comparable
with that in the large animals described previously.
Scaffold in bone tissue engineering
Currently, the scaffolds are mainly made from (1)
synthetic materials including inorganic substances
(hydroxyapatite and calcium phosphate) and organic
substances (polylactic acid and polyglycolic acid); (2)
naturally derived materials including calcined bone,
demineralized bone matrix and deproteined bone
matrix and other natural coral materials (Liu et al.
2008, Sundelacruz et al. 2009, Cordonnier et al.
2010). The synthetic materials are easy to prepare and
not restricted by sources. Recently, with the
development of a rapid prototyping technique (He et
al. 2010), great progress has been made in research
into synthetic materials. However, the effects of the
decomposition of organic synthetic materials on cells
and the degradation of inorganic materials limit the
wide application of scaffolds in clinical practice (Liu
et al. 2008, Sundelacruz and Kaplan 2009, He et al.
2010). Numerous studies have show that bio-derived
bone after processing not only has low antigenicity
but can induce osteogenesis, which facilitates the
adherence and growth of seed cells. The development
of these materials improves the porosity, pore
communication and pore size which are the key
problems in the preparation of biomimetic materials.
In addition, these materials have a rich source, are
easy to prepare and are less costly (Liu et al. 2008,
Revell and Athanasiou 2009, Sundelacruz and Kaplan
2009, Bedi et al. 2010, Cordonnier et al. 2010, He et
al. 2010). With the development of manufacturing
technology and discipline, scaffolds of bio-derived
bone will play a critical role in bone tissue
engineering.
In the present study, the ectopic bones were
developed by our institute. These bones preserve the
favourable pore structure of cancellous bone and have
low antigenicity. They have been applied as non cell
scaffolds in more than 1000 patients in our
department, achieving favourable outcomes (Hu and
Lu 1990, Yuan et al. 1999, 2003a, b). But evidence
on these bones as cell scaffolds is insufficient. In the
present study, they were used as cell scaffolds and
had good biocompatibility with MSCxj cells. In
addition, the cells were well adherent to these
scaffolds and acceptable ectopic osteogenesis was
observed after implantation of cells and scaffolds.
Our results demonstrated these ectopic bones were
good scaffolds in bone tissue engineering. However,
the degradation of these scaffolds was relatively slow
and obvious degradation was not observed even
12 weeks after implantation. Therefore, more studies
are required to investigate the degradation of ectopic
bones in the bone tissue engineering.
Inoculation of seed cells and scaffold
To tightly and evenly attach numerous seed cells to
scaffolds is a problem in bone tissue engineering.
Currently, only two methods are adopted (Li et al.
2005, Liu et al. 2008): (1) repeated precipitation,
where the cell suspension is dropped onto the
scaffolds and then incubated in culture medium in an
incubator. These procedures are repeated several
times. (2) negative adsorption, where the scaffolds are
put into a relative vacuum container containing cell
suspension of high density. After negative adsorption,
the seed cells are adherent to the scaffolds (Zou et al.
2009, Richardson et al. 2010). However, the negative
pressure is hard to regulate and practice should be
performed before application. In the recent years, a
bioreactor has been applied in the culture of BMSCs
which facilitates the large-scale production of seed
cells. In addition, a thermostatic oscillation incubator
is used for the inoculation of seed cells and scaffolds.
These developments obviously play critical roles in
molecular and cellular bioengineering.
CONCLUSION
The present study aimed to investigate the feasibility
of MSCxjs as seed cells in bone tissue engineering.
Repeated precipitation was applied and ectopic
osteogenesis of MSCxjs detected. For small scaffolds,
our method is favourable and seed cells could be
evenly distributed on and in the scaffolds. Therefore,
this method may be applied in thin cancellous bone
materials and small granular materials.
REFERENCES
Bauer Tw, Muschler GF. Bone graft materials; an overview of the basic science. Clin Orthop. 371: 10-14, 2000.
[PubMed]
Bedi A, Feeley BT, Williams RJ. 3rd Management of articular cartilage defects of the knee. J Bone Joint Surg Am. 92: 994-1009, 2010.
[CrossRef]
[PubMed]
Byun IS, Sarkar SK, Anirban Jyoti M, Min YK, Seo HS, Lee BT, Song HY. Initial biocompatibility and enhanced osteoblast response of Si doping in a porous BCP bone graft substitute. J Mater Sci Mater Med. 21: 1937-1947, 2010.
[CrossRef]
[PubMed]
Cordonnier T, Layrolle P, Gaillard J, Langonne A, Sensebe L, Rosset P, Sohier J. 3D environment on human mesenchymal stem cells differentiation for bone tissue engineering. J Mater Sci Mater Med. 21: 981-987, 2010.
[CrossRef]
[PubMed]
He H, Cao J, Wang D, Gu B, Guo H, Liu H. Gene-modified stem cells combined with rapid prototyping techniques: a novel strategy for periodontal regeneration. Stem Cell Rev. 6: 137-141, 2010.
[CrossRef]
[PubMed]
Hesse E, Kluge G, Atfi A, Correa D, Haasper C, Berding G, Shin HO, Viering J, Langer F, Vogt PM, Krettek C, Jagodzinski M. Repair of a segmental long bone defect in human by implantation of a novel multiple disc graft. Bone. 46: 1457-1463, 2010.
[CrossRef]
[PubMed]
Hodgkinson T, Yuan XF, Bayat A. Adult stem cells in tissue engineering. Expert Rev Med Devices. 6: 621-640, 2009.
[CrossRef]
[PubMed]
Hu YY, Lu YP. Experimental studies on the repair of bone defect through bone xenograft (in Chinese). Chin J Orthop. 10: 33-36, 1990.
Itescu S, Schuster MD, Kocher AA. New directions in strategies using cell therapy for heart disease. J Mol Med. 81: 288-296, 2003.
[PubMed]
Jukes JM, van Blitterswijk CA, de Boer J. Skeletal tissue engineering using embryonic stem cells. J Tissue Eng Regen Med. 4: 165-180, 2010.
[CrossRef]
[PubMed]
Li WJ, Tuli R, Okafor C, Derfoul A, Danielson KG, Hall DJ, Tuan RS. A three-dimensional nanofibrous scaffold for cartilage tissue engineering using human mesenchymal stem cells. Biomaterials. 26: 599-609, 2005.
[CrossRef]
[PubMed]
Liu Y, Ramanath HS, Wang DA. Tendon tissue engineering using scaffold enhancing strategies. Trends Biotechnol. 26: 201-209, 2008.
[CrossRef]
[PubMed]
Lv R, Yang G. Comparisons between plastic undecalcified sections, paraffin-embedded sections and gelatin immersed sections (in Chinese). Chin J Clin Exp Pathol. 15: 464-465, 1999.
Lv R, Xu XZ, Wang J. Sectioning and staining of plastic-embedded and undecalcified sections (in Chinese). Chin J Clin Exp Pathol. 18 :342, 2002.
Lv R, Wang J, Xu XZ. Bone histology and image analysis of plastic-embedded and undecalcified sections (in Chinese). Chin J Clin Exp Pathol. 22: 369-370, 2006.
Nauta AJ, Westerhuis G, Kruisselbrink AB, Lurvink EGA, Willemze R, Fibbe WE. Donor-derived mesenchymal stem cells are immunogenic in an allogeneic host and stimulate donor graft rejection in a nonmyeloablative setting. Blood. 108: 2114-2120, 2006.
[CrossRef]
[PubMed]
Poncelet AJ, Vercruysse J, Saliez A, Gianello P. Although pig allogeneic mesenchymal stem cells are not immunogenic in vitro, intracardiac injection elicits an immune response in vivo. Transplantation. 83: 783-790, 2007.
[CrossRef]
[PubMed]
Ren HY, Zhao QJ, Xing W. Differentiation of human umbilical cord derived mesenchymal stem cells into low immunogenic and functional hepatocyte-like cells in vitro (in Chinese). Zhongguo Yi Xue Ke Xue Yuan Xue Bao 32:190-194, 2010.
[PubMed]
Revell CM, Athanasiou KA: Success rates and immunologic responses of autogenic, allogenic, and xenogenic treatments to repair articular cartilage defects. Tissue Eng Part B Rev. 15: 1-15, 2009.
[CrossRef]
[PubMed]
Richardson SM, Hoyland JA, Mobasheri R, Csaki C, Shakibaei M, Mobasheri A. Mesenchymal stem cells in regenerative medicine: opportunities and challenges for articular cartilage and intervertebral disc tissue engineering. J Cell Physiol. 222: 23-32, 2010.
[CrossRef]
[PubMed]
Spees JL, Gregory CA, Singh H, Tucker HA, Peister A, Lynch PJ, Hsu SC, Smith J, Prockop DJ. Internalized antigens must be removed to prepare hypoimmunogenic mesenchymal stem cells for cell and gene therapy. Mol Ther. 9: 747-756, 2004.
[CrossRef]
[PubMed]
Sundelacruz S, Kaplan DL. Stem cell- and scaffold-based tissue engineering approaches to osteochondral regenerative medicine. Semin Cell Dev Biol. 20: 646-655, 2009.
[CrossRef]
Teng Y, Hu YY, Wang HG. Study of hTERT activating telomerase in human bone marrow mesenchyme stem cells (in Chinese). Orthop J China. 7: 1253-1256, 2007a.
Teng Y, Hu YY, Wang Z. Differentiation of human bone marrow-derived mesenchymal stem cells into chondrocytes in vitro (in Chinese). Chin J Exp Surg. 12: 17-19, 2007b.
Yuan Z, Zhao L, Hu YY. Combined use of rhBMP 2/BCB and periosteum in repairing segmental defects in radii of rabbits. Chin J Orthop. 19: 45-49+65, 1999.
Yuan Z, Hu YY, Li MQ. Therapeutic effect of anti-infective reconstituted bone xenograft on osteomyelitis in proximal tibia of rabbits (in Chinese). Chin J Orthop. 23: 230-234, 2003a.
Yuan Z, Zhao L, Hu YY. Anti-infective reconstituted bone xenograft used for primary bone grafting to repair contaminated defect in the radius in dogs (in Chinese). Chin J Traumatol. 6: 86-90, 2003b.
[PubMed]
Zhao CG, Hu YY, Lv R. The osteoinductivity and the dose-effect relationship with implantation of reconstituted bone xenograft: experimental study (in Chinese). Chin J Surg. 36: 627-629, 1998.
[PubMed]
Zou XH, Cai HX, Yin Z, Chen X, Jiang YZ, Hu H, Ouyang HW. A novel strategy incorporated the power of mesenchymal stem cells to allografts for segmental bone tissue engineering. Cell Transplant. 18: 433-441, 2009.
[CrossRef]
[CrossRef]
[PubMed]
[PubMed]
|
BACK
|





