Journal of APPLIED BIOMEDICINE
ISSN 1214-0287 (on-line)
ISSN 1214-021X (printed)
Volume 9 (2011), No 3, p 129-141
DOI 10.2478/v10136-011-0001-8
Effects of SDF-1alpha/CXCR4 on vascular smooth muscle cells and bone marrow mesenchymal cells in a rat carotid artery balloon injury model
Wen-Wei Cai, Ning-Yuan Fang, Jing Sheng, Shao-Jun Ma, Zhi-Hui Chen
Address: Ning-Yuan Fang, No. 639, Zhi-Zao-Ju Road, Shanghai 200011, P. R. China
funfeng111@gmail.com
Received 20th December 2010.
Revised 10th February 2011.
Published online 7th April 2011.
Full text article (pdf)
Abstract in xml format
Summary
Key words
Introduction
Materials and methods
Results
Discussion
References
SUMMARY
Bone marrow mensenchyme cells(BMSCs) can differentiate into endothelial progenitor cells which then migrate to injured sites for the repair of
neointima, and stromal cell-derived factor-1alpha (SDF-1alpha) can mediate the migration of CXCR4 expressing stem/progenitor cells to injured
sites for repair. Protein and mRNA expression of SDF-1alpha and CXCR4 were determined by RT-PCR, Western blot and ELISA. Immediately after common
carotid artery balloon injury, the mRNA expression of SDF-1alpha in vascular smooth muscle cells(VSMCs) first increased and then decreased 7 days
later. VSMCs transfected with SDF-1alpha siRNA did not express SDF-1alpha mRNA, but after transfection with SDF-1alpha siRNA, the SDF-1alpha
content in injured VSMCs gradually returned to the baseline level. Normal BMSCs rarely expressed CXCR4 mRNA, but the CXCR4 mRNA expression on
BMSCs increased significantly 4 days after common carotid artery injury and was maintained. The migration of BMSCs after artery injury was
enhanced when compared with normal BMSCs, but SDF-1alpha siRNA transfection of VSMCs and AMD3100 treatment remarkably decreased the chemotaxis of
BMSCs to VSMCs and SDF-1alpha, respectively. We conclude that the SDF-1alpha/CXCR4 axis plays an important role in the migration of BMSCs after
balloon injury and can ultimately cause abnormal proliferation of the intima.
KEY WORDS
bone marrow mensenchyme cells; vascular smooth muscle cells; stromal cell-derived factor-1alpha
INTRODUCTION
Vascular smooth muscle cells (VSMCs) have been
found to be related to atherosclerosis, restenosis after
balloon injury (percutaneous transluminal coronary
angioplasty and coronary stent implantation) and
hypertension induced vascular remodeling (Schwartz
1994). Experiments confirm that, after vascular
injury, mature VSMCs can switch from contractile
phenotype to synthetic phenotype, and acquire the
capability not only to proliferate and migrate but also
to secrete numerous cytokins (Bochaton-Piallat et al.
1996). Bone marrow mensenchyme cells (BMSCs)
have multipotentiality, and can differentiate into
endothelial progenitor cells after stimulation and
migrate from bone marrow to injured sites where they
are involved in the repair of neointima through
colonization and proliferation.
Stromal cell-derived factor-1alpha (SDF-1alpha) is a small
cytokine belonging to the chemokine family that is
officially designated Chemokine (C-X-C motif)
ligand 12 (CXCL12). SDF-1alpha can direct the
adherence of inflammatory cells in blood to
endothelial cells and mediate the migration of
inflammatory cells into subintima where they play an
important role (Gleichmann et al. 2000). CXCR4 is a
G protein coupled receptor of SDF-1alpha with
seven-transmembrane domains and is widely
expressed in numerous cells and non-haematopoietic
organs. Studies have demonstrated that CXCR4 plays
an important role in the migration of haematopoietic
stem cells, and is also involved in cancer metastasis,
human immunodeficiency syndrome, inflammation
and ischemia (Kucia et al. 2004b). In the present
study, a rat carotid artery balloon injury model was
established, and then BMSCs and VSMCs were
obtained and maintained in vitro. The effects of
SDF-1alpha/CXCR4 on the proliferation and migration of
these cells were determined.
MATERIALS AND METHODS
Animals, surgical procedures and cell culture
Male Sprague Dawley (SD) rats, 8-week-old,
weighing 286±14.3 g, were purchased from the SLAC
Laboratory Animal CO., LTD. (Shanghai, China) and
housed in a temperature-controlled environment
(22-24 °C) with a light dark regime of 12/12 hrs. All
procedures were approved by the local ethics
committee of the School of Medicine, Shanghai
Jiao-Tong University (No. JYLL-10002) and animal
use conformed to the National Institute of Health
guidelines on the ethical use of animals and to the
guidelines for caring for laboratory animals issued by
the Ministry of Science and Technology of the
People's Republic of China.
A rat common carotid artery balloon injury model
was established as described in Tulis et al. (2001).
Briefly, the rat was intraperitoneally anaesthetized
with 2.5% pentobarbital sodium (40 mg/kg) and fixed
in the supine position. A midline incision was made
in the neck, and then the left common carotid artery
and the bifurcation of internal and external carotid
arteries were exposed. A "V" incision was made on
the external carotid artery followed by insertion of a
2F thrombotic balloon catheter (Edwards
Lifesciences, USA) deeply into the common carotid
artery, and then the balloon was dilated by infusing
0.10-0.15 ml of normal saline. The catheter was
subsequently drawn back to cause damage to the
intima. Then, the normal saline was withdrawn and a
catheter inserted to the common carotid artery. The
procedures were performed twice in order to
completely peel off the intima. Finally, an incision
suture was performed. The rat was given ad libitum
access to food and water.
Culture of VSMCs from common carotid artery
VSMCs from the common carotid artery were
cultured using the method previously described by
Ross et al. (1971). Male SD rats were sacrificed 0,
1 d, 4 d, 7 d, 14 d, and 1 m after common carotid
artery balloon injury (S0, S1d, S4d, S7d, S14d and S1m)
and the injured common carotid arteries were
obtained. SD rats in the control group (healthy rats)
were housed for 2 weeks and sacrificed followed by
removal of the common carotid arteries. The arteries
were washed with normal saline and opened
longitudinally. The intima was removed and the
remaining arteries were rinsed with PBS and cut into
1 mm2 pieces. These pieces were maintained in
DMEM (high glucose) (Gibco, USA) containing 10%
FBS at 37 °C in humidified air with 5% CO2
(incubator; Viscon Systems Sdn Bhd, Malaysia).
Twenty four hour later, when adherence was
observed, the medium was supplemented followed by
culture. The medium was refreshed twice weekly.
Culture of BMSCs
BMSCs were cultured according to the method
previously described by Law et al. (1996) and Barry
and Murphy (2004). SD rats were sacrificed using
this method, and bilateral femurs were obtained. The
attached muscle and fascia were removed and femurs
were fractured in the middle. The femurs were
flushed with PBS and washing fluid was filtered
through a filter (40 microm, BD Falcon) followed by
centrifugation at 1500 rpm for 5 min at room
temperature. The cells were resuspended in 1 ml of
DMEM (low glucose) and a single cell suspension
was prepared. The cells were maintained in dishes at
37 °C in humidified air with 5% CO2. When
adherence was observed (48-72 h later), the adherent
red blood cells were removed by PBS washing
followed by further culture. The medium was
refreshed twice weekly. The acquired cells were
detected with flow cytometry; the results showed that
these cells were positive for CD44 and negative for
CD34 and CD45.
Transfection of SDF-1alpha siRNA into VSMCs of rats with common carotid artery injury
The 3rd passage VSMCs from rats sacrificed at
different time points were seeded in 35 mm dishes at
a density of 3x105/ml. SDF-1alpha siRNA (Invitrogen,
China) was mixed with FuGENE 6 Transfection
Reagent (Roche, Swiss) at a ratio of 1:3 (v/v)
followed by incubation for 15 min. Then, this mixture
was added to the cells followed by incubation for
24 h. The cells were then harvested (Si0, Si1d, Si4d,
Si7d, Si14d and Si1m).
Detection of cell proliferation by MTT assay
The 3rd passage VSMCs of healthy rats, of those with
common carotid artery injury and VSMCs tranfected
with SDF-1alpha siRNA were digested with 0.25%
trypsin-EDTA (Amresco, USA) and a single cell
suspension was prepared. The cells were seeded in a
96-well plate (100 microl/well) at a density of 2x105/ml
followed by incubation in an incubator. When
adherence was observed, synchronization was
performed for 24 h, and MTT solution (5 g/l) (Sigma
USA) was added to the cells (20 microl/well) followed by
incubation for 4 h. Then, the medium was removed
and DMSO (Sigma, USA) was supplemented
(100 microl/well) followed by incubation for 10 min with
continuous shaking until crystals resolved.
Absorbance was determined with a microplate reader
(VARIO Thermo Electron Technology, Japan) at
570 nm. The absorbance reflects the proliferation of
VSMCs. The experiment was performed in triplicate
and data were averaged. The experiment was repeated
six times.
Detection of SDF-1alpha and CXCR4 mRNA expression in VSMCs and BMSCs by RT-PCR
Total RNA was extracted from the 3rd passage
VSMCs and BMSCs with TRIzol (Invitrogen, Life
Technologies, China), and RT-PCR was performed to
detect the mRNA expression of SDF-1alpha in VSMCs
and CXCR4 in BMSCs. The primers were
synthesized by Shanghai Sangong Co., Ltd., China. SDF-1alpha primers (381 bp):
Forward:
5'-CCAATCAGAAATGGGAACAAGA-3'
Reverse:
5'-GTAGGAGGCTTACAGCACGAA-3'
CXCR4 primers (267 bp)
Forward: 5'-GTGGGCAATGGGTTGGTAAT-3'
Reverse:
5'-GGTGGCGTGGACAATGGCAAGGTAG-3'
The cDNA was denatured at 94 °C for 1 min,
followed by 34 cycles of amplification. Each cycle
consisted of denaturation at 94 °C for 30 sec,
annealing at 55 °C for 30 sec, and extension at 72 °C
for 45 sec, with an additional extension for 5 min at
72 °C. The quality of the PCR products was
determined by electrophoresis.
Detection of CXCR4 expression in BMSCs by Western blot
Total proteins were extracted from the 3rd passage
BMSCs and protein concentration in the supernatant
was measured spectrophotometrically at 595 nm.
Then, 40 microg of proteins were loaded onto SDS
polyacrylamide gel for electrophoresis (Invitrogen,
China), and transferred onto PVDF membranes
(Millipore, USA). The membranes were incubated
with rabbit anti-mouse CXCR4 antibody (1:500;
eBioscience, USA) and goat anti-mouse beta actin
antibody (1:1000; Santa Cruz, USA) overnight at
4 °C. Then, the membranes were incubated with
secondary antibodies (donkey anti-rabbit antibody,
800DX 1:5000 eBioscience, USA; donkey anti-goat
antibody, 700DX 1:2000, Sigma Chemical
Company, USA) for 1 h, followed by detection with
an Infrared Fluorescence Imaging and Analyzing
System (Odyssey v1.2) (FIAS, Odyssay LI-COR,
USA).
Enzyme-linked immunosorbent assay of plasma SDF-1alpha
The plasma level of SDF-1alpha was determined by the
enzyme-linked immunosorbent assay (ELISA) using
an ELISA kit (R&D; system, Inc., USA) according to
the manufacturer's instructions.
Detection of SDF-1alpha in the supernatants of VMSCs by ELISA
The 2nd passage VMSCs from healthy rats and injured
rats sacrificed at different time points were seeded in
a dish at a density of 2x105/100 mm. VMSCs in the
Si group were transfected with SDF-1alpha siRNA. Then,
1 ml of supernatants was collected when 90%
confluence was observed and stored at -80 °C for
later use. The SDF-1alpha content was determined
according to The manufacturer's instructions (Human
SDF-1alpha Immunoassay, USA).
Detection of cell migration
Cells were divided into six groups (Table 1). Cell
migration was determined according to the method
previously described by Dowell et al (2003).
Polycarbonate membranes with 8 microm pore size were
inserted into the chambers (Corning, USA). VMSCs
were glucose deprived for 24 h and washed with PBS.
Cells were digested and re-suspended in DMEM (low
glucose) containing 0.4% FBS. Cell density was
adjusted to 1x106/ml. Cells with and without 200
ng/ml AMD3100 (Octahydrochloride, Sigma, USA)
treatment for 30 min were added into the upper
chamber. Then, 500 microl of DMEM (low glucose)
containing 0.4% FBS and with or without 100 ng/ml
SDF-1alpha (Peprotech, UK) were added to the lower
chamber. The injured VSMCs were collected when
the SDF-1alpha content in the supernatant reached
maximal level (P1d). When 80-90% confluence was
observed, the cells were harvested, washed, digested and resuspended in DMEM (low glucose) containing
0.4% FBS. Cell density was adjusted to 1x106/ml, and
500 l of cell suspension were added to the lower
chamber. The transwell chamber was incubated for
6 h. The upper chamber was taken out and cells were
scrubbed with cottons. Then, the cells were washed
with PBS and fixed in ice cold 95% ethanol for
10 min. Staining with hematoxylin was performed for
15 min and cells were counted under a light
microscope (x200). Five fields in each section were
randomly selected and data were averaged. The
experiment was repeated 3 times.
Table 1. Grouping and processing of VSMCs and BMSCs.
| Normal group |
S group |
S+A group |
P group |
P+A group |
P+siRNA group | |
NMSC+S |
NMSC+S+A |
NMSC+P1dSMC |
NMSC+P1dSMC
+A |
NMSC+P1dSMC
+siRNA | |
S0MSC+S |
S0MSC+S+A |
S0MSC+P1dSMC |
S0MSC+P1dSMC
+A |
S0MSC+P1dSMC
+siRNA | |
S1dMSC+S |
S1dMSC+S+A |
S1dMSC+P1dSMC |
S1dMSC+P1dSMC
+A |
S1dMSC+P1dSMC
+siRNA | | NMSC |
S4dMSC+S |
S4dMSC+S+A |
S4dMSC+P1dSMC |
S4dMSC+P1dSMC
+A |
S4dMSC+P1dSMC
+siRNA | |
S7dMSC+S |
S7dMSC+S+A |
S7dMSC+P1dSMC |
S7dMSC+P1dSMC
+A |
S7dMSC+P1dSMC
+siRNA | |
S2wMSC+S |
S2wMSC+S+A |
S2wMSC+P1dSMC |
S2wMSC+P1dSMC
+A |
S2wMSC+P1dSMC
+siRNA | |
S1mMSC+S |
S1mMSC+S+A |
S1mMSC+P1dSMC |
S1mMSC+P1dSMC
+A |
S1mMSC+P1dSMC
+siRNA |
S group: SDF-1alpha treatment group; S+A group: SDF-1alpha+AMD3100 treatment group; P group: injured VSMCs group; P+A group:
injured VSMCs+AMD3100 treatment group; P+siRNA group: injured VSMCs with SDF-1alpha siRNA transfection group;
NMSC: normal BMSCs; S0MSC: BMSCs collected immediately after injury; S1dMSC: BMSCs collected 1 d after injury;
S4dMSC: BMSCs collected 4 d after injury; S7dMSC: BMSCs collected 7 d after injury; S2wMSC: BMSCs collected 2
weeks after injury; S1mMSC: BMSCs collected 1 month after injury;
S: SDF-1alpha 100 ng/ml; A: AMD3100 200 ng/ml.
Statistical analysis
Experiments were performed at least thrice and data
presented as the mean ± standard deviation (SD).
Statistical analysis was performed with SPSS version
13.0 (SPSS Inc., Chicago, USA). The unpaired t test
was employed for comparison between the two
groups and one-way analysis of variance (ANOVA)
among multiple groups. Data were statistically
evaluated at the significance level of 2alpha=0.05.
RESULTS
Proliferation of VSMCs
An MTT assay was performed in the VSMCs from
rats with or without common carotid artery injury.
Results showed that the proliferation of VSMCs from
rats with common carotid artery injury was
significantly more active than in those from healthy
rats (statistically significant) (Table 2).
mRNA expression of SDF-1alpha in VSMCs
PCR was performed to detect the mRNA expression
of SDF-1alpha in VSMCs; the results showed the mRNA
expression of SDF-1alpha was markedly decreased in
normal VSMCs when compared with that in rats with
common carotid artery injury. SDF-1alpha mRNA
expression increased immediately after injury and the
enhanced expression of SDF-1alpha was more evident in
the S1d, S4d and S7d groups (Fig. 1A). However, the
mRNA expression of SDF-1alpha was not detectable in
VSMCs transfected with SDF-1alpha siRNA (Fig. 1B).
Table 2. Proliferation of VSMCs detected by MTT assay.
| N
group |
S group |
Si group | | S0 |
S1d |
S4d |
S7d |
S2w |
S1m |
Si0 |
Si1d |
Si4d |
Si7d |
Si2w |
Si1m | | 0.405±0.011 |
0.487±0.033* |
0.574±0.098* |
0.698±0.075* |
0.805±0.100* |
1.317±0.112* |
1.540±0.173* |
0.497±0.068* |
0.593±0.096* |
0.684±0.116* |
0.841±0.120* |
1.392±0.109* |
1.576±0.097* |
* statistically significant versus controls
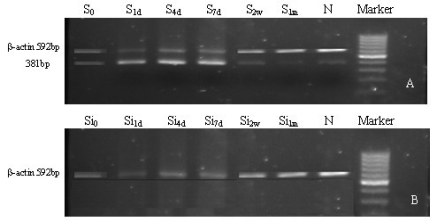
Fig. 1. mRNA expression of SDF-1alpha in VSMCs. A) mRNA expression of SDF-1alpha in VSMCs from rats with common carotid
artery injury, B) mRNA expression of SDF-1alpha in injured VSMCs with SDF-1alpha siRNA transfection.
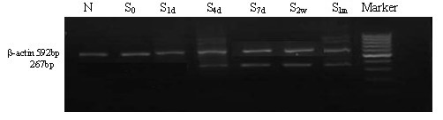
Fig. 2. mRNA expression of CXCR4 in BMSCs from rats with common carotid artery injury.
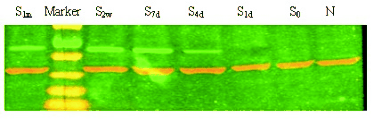
Fig. 3. Protein expression of CXCR4 in BMSCs of SD rats with common carotid artery injury.
mRNA expression of CXCR4 in BMSCs
PCR was performed to detect the mRNA expression
of CXCR4 in BMSCs and the results indicated that
CXCR4 mRNA expression was hardly detectable in
NMSCs. However, 4 days after injury, the mRNA
expression of CXCR4 increased (Fig. 2).
Protein expression of CXCR4 in BMSCs
The CXCR4 expression in BMSCs was determined
by western blot and results did not show CXCR4
protein expression in NMSCs. However, 4 days after
injury, the protein expression of CXCR4 increased
(Fig. 3).
Plasma level of SDF-1alpha after intimal injury
As shown in Table 3, the plasma level of SDF-1alpha
after intimal injury was markedly increased and
reached the maximum 1 day after injury (statistically
significant) followed by a rapid decrease to the
baseline level on day 7. The administration of
AMD3100 did not affect the plasma level of SDF-1alpha.
SDF-1alpha content in the supernatants of VSMCs
ELISA was performed to detect the SDF-1alpha content
in the supernatants of VSMCs. Results showed that
the SDF-1alpha content in normal VSMCs was lower than
that in injured VSMCs. The SDF-1alpha content
increased immediately after common carotid artery
injury and reached a maximal level 1 day after injury.
However, 7 days after injury, the SDF-1alpha content
decreased to a normal level. In VSMCs transfected
with SDF-1alpha siRNA; the SDF-1alpha content remained at
a baseline level (Table 4 and 5).
Effects of VSMCs on the migration of BMSCs
The effect of VSMCs on the chemotaxis of BMSCs
was determined by a Transwell chamber assay. Under
normal conditions, there were no chemokines in the
lower chamber and no obvious chemotaxis of normal
BMSCs was found. When 100 ng/ml SDF-1alpha was
added to the lower chamber, the chemotaxis of
BMSCs from injured and normal rats was markedly
enhanced when compared with BMSCs without
SDF-1alpha treatment (statistically significant).
Furthermore, the chemotaxis of BMSCs from injured
rats was more evident than that from normal rats
(statistically significant). When 200 ng/ml AMD3100,
an antagonist of CXCR4, was supplemented to the
upper chamber followed by incubation for 30 min, the
chemotaxis of BMSCs was dramatically inhibited.
When the injured VSMCs were added to the lower
chamber, the chemotaxis of BMSCs was also noted
and it was more obvious than that induced by SDF-1alpha
(statistically significant). In addition, after AMD3100
treatment of BMSCs or SDF-1alpha siRNA transfection
of VSMCs, the chemotaxis of BMSCs induced by
VSMCs declined, and it was more evident than that
induced by SDF-1alpha after AMD3100 treatment
(statistically significant) (Fig. 4A-F) (Table 6).
DISCUSSION
BMSCs have been a hot topic in studies on the
treatment of diseases, especially cardiovascular
diseases (acute myocardial infarction). Increasing
evidence has shown the efficacy of BMSCs. The goal
of treatment with BMSCs is to achieve normal and
healthy tissues and maintain the whole function.
Currently, the treatment of myocardial infarction with
stem cells can be performed in three ways: (1) Stem
cell transplantation: the mature stem cells are
harvested and injected into the area with infarction;
(2) Stem cell mobilization: the ability of stem cells to
repair the injured heart is enhanced through
mobilization of stem cells from bone marrow, and (3)
Local treatment with cytokines: stem cells (stem cells
in bone marrow and circulation) can regulate the
expression of cytokines and growth factors enhancing
the ability to repair the injured heart.
Stem cells are characterized by the ability to
renew themselves and differentiate into a diverse
range of specialized cell types. Tissue-committed
stem cells (TCSCs) are cells with multiple
differentiation potential in mature individuals.
Currently, BMSCs have been found to have the
ability to transform. Analysis of the cell cycle of
BMSCs shows that about 20% of BMSCs are in the
quiescent phase (G0 phase), which suggests that
BMSCs have a potent ability to proliferate. The
number of BMSCs may be increased by 2-4 fold at
each passage. Minguell et al. (2001) speculated that
BMSCs are a non-haematopoiesis derived cell
population, and have a high requirement for nutrition.
The proportion of BMSCs in the bone marrow is
extremely low and accounts for about 0.001-0.01% of
cells in bone marrow. Therefore, the application of
BMSCs should be realized through in vitro culture
and amplification (Pittenger et al. 1999). Numerous
studies have demonstrated the effectiveness of stem
cells in ischemic injury (Orlic et al. 2002, Kucia et al.
2004a, Mathur and Martin 2004). Bone marrow is the
main stem cell bank in the body, and stem cells in
bone marrow can be activated in an appropriate
environment, and motivated into peripheral blood.
Ratajczak et al. (2004) and Papayannopoulou (2000)
speculated that bone marrow not only stores a lot of
haematopoietic stem cells (HSCs) but also provides
space for the storage of TCSCs from peripheral blood, including stem cells in the muscle, liver, brain and
heart. After stress or injury (such as acute myocardial
infarction), TCSCs in the peripheral blood increase
significantly and are involved in the repair of injured
tissues.
Table 3. Plasma level of SDF-1alpha after intimal injury (x±s, ng/ml, n=12 per group).
|
Normal
group
(N group) |
Common carotid artery injury group (S group) | |
S0 |
S1d |
S4d |
S7d |
S1m |
S3m | | SDF-1alpha (ng/ml) |
0.312±
0.006 |
0.885±
0.022* |
1.328±
0.009* |
1.119±
0.013* |
0.323±
0.005 |
0.320±
0.006 |
0.309±
0.056 |
symbols as in Table 2
Table 4. SDF-1alpha content in the supernatants of injured VSMCs from rats sacrificed at different time points.
|
Normal
group
(N group) |
Common carotid artery injury group (S group) | |
S0 |
S1d |
S4d |
S7d |
S2w |
S1m | | SDF-1alpha (ng/ml) |
0.692±
0.047 |
0.324±
0.070* |
1.897±
0.058* |
1.519±
0.063** |
0.711±
0.068 |
0.706±
0.038 |
0.676±
0.044 |
* statistically significant as compared with controls; ** statistically significant vs NMSC group
Table 5. SDF-1alpha content in the supernatants of injured VSMCs transfected with SDF-1alpha siRNA from rats sacrificed at
different time points.
|
Normal
group
(N group) |
siRNA transfection group (Si group) | |
Si0 |
Si1d |
Si4d |
Si7d |
Si2w |
Si1m | | SDF-1alpha (ng/ml) |
0.692±
0.047 |
0.726±
0.057 |
0.742±
0.061 |
0.703±
0.024 |
0.685±
0.037 |
0.715±
0.049 |
0.700±
0.041 |
symbols as in Table 4
Currently, the mechanism underlying stem cell
mobilization is still poorly understood, and may
involve numerous cytokines, a variety of proteinases
(such as elastase, matrix metalloproteinase and
protease G), adhesion molecules and vascular
endothelial cells in an haematopoietic
microenvironment. These factors interact with each
other and finally result in the release of HSCs from
bone marrow to peripheral blood (Lapidot and Petit
2002). Many cytokines have been found to stimulate
the motivation of BMSCs (mainly HSCs) to
peripheral blood including G-CSF, GM-CSF, SCF
VEGF, IL-8 and SDF-1 (Misao et al. 2006). The
SDF-1alpha could enhance the binding of tissues
expressing SDF-1alpha to CXCR4 on the circulation
progenitor cells. In addition, the number of
CD34+CXCR4+ cells is related to the SDF-1alpha level
after CP/CPB. However, this relationship was not
noted before CP/CPB and 4 days after CP/CPB.
These findings suggest that the motivation of
progenitor cells and production of key cytokines
occurred a few hours after injury. Misao et al (2006)
also demonstrated that AMD3100, an antagonist of
CXCR4, could block the interaction between
CXCR4+ cells and SDF-1alpha, and also counteract the
improvement of myocardial function by G-CSF in
acute myocardial infarction.
VSMCs have been shown to exist in two
phenotypic states: contractile phenotype and synthetic
phenotype. Normal VSMCs are mainly characterized
by a differentiated phenotype. When vessels are injured or these cells are treated with certain growth
factors, vasoactive substances or neurotransmitters in
vitro, the phenotype is switched from differentiated to
dedifferentiated and these cells are able to proliferate.
Zhang et al. (2008) found that, after culture of vessels
with endothelial cell injury, the VSMCs started to
proliferate and the proliferation increased over time.
Not only were VSMCs switched from contractile
phenotype to synthetic phenotype, but an obvious
proliferation of VSMCs was noted. These findings are
also observed in atherosclerosis plaque and restenosis
after percutaneous transluminal angioplasty. In the
present study, the endothelial cells of the common
carotid artery were injured and VSMCs were
maintained in vitro. These VSMCs were
characterized by synthetic phenotype and active
proliferation. In addition, the proliferation of injured
VSMCs was not affected by SDF-1alpha siRNA
transfection.
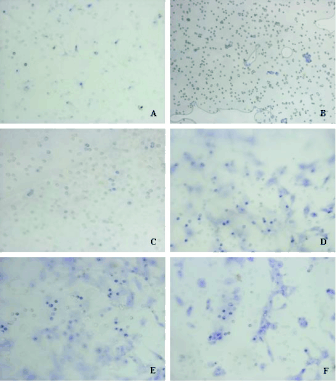
Fig. 4. Effects of VSMCs on the migration of BMSCs. A) normal BMSCs (NMSC group), B) SDF-1alpha treatment group (S
group), C) SDF-1alpha+AMD3100 treatment (S+A group), D) intervention with injured VSMCs (P group), E) intervention with
injured VSMCs and AMD3100 (P+A group), F) injured VSMCs transfected with SDF-1alpha siRNA (P+siRNA group).
After injury, SDF-1 expression can be detected in
the injured intima and media, and CXCR4 expression
is also detectable in the intima. Gao and Li (2007)
transplanted the abdominal aorta of Wistar rats to SD
rats, and the results showed that the proliferation of
neointima in the abdominal aorta graft of SD rats was
almost completely suppressed after SDF-1 antibody
treatment. Furthermore, the thickness of neointima
was significantly related to the SDF-1 level.
Our study showed that the mRNA expression of
SDF-1alpha increased immediately after common carotid
artery injury and that the increase of mRNA
expression continued until 4 days after injury.
However, the mRNA expression of SDF-1alpha 7 days,
2 weeks and 1 month after injury was similar to that
before injury. ELISA was performed to detect the
SDF-1alpha content in the plasma and in the cell culture
supernatants respectively, and the results showed that
the SDF-1alpha content increased immediately after
common carotid artery injury both in vivo and in
vitro. The SDF-1alpha content reached a maximal level
1 day after injury and returned to normal level 7 days
after injury. There was a relationship between
SDF-1alpha expression in VSMCs and SDF-1alpha content in
the supernatants, and our results were consistent with previous reports (Suda et al. 1987). These findings
suggest that injury stimulates the transcription and
translation of SDF-1alpha and finally leads to increased
protein expression of SDF-1alpha.
Table 6. Effect of VSMCs on the chemotaxis of BMSCs determined by Transwell chamber assay.
| Group |
Number of cells | | NMSC group |
6.21±0.35 | | SDF-1alpha treatment (100 ng/L S group) |
NMSC+S
S0MSC+S
S1dMSC+S
S4dMSC+S
S7dMSC+S
S2wMSC+S
S1mMSC+S |
13.42±0.58*
22.67±0.61**#
25.71±0.34**#
24.85±0.64**#
25.19±0.73**#
26.01±0.26**#
25.85±0.17**# | | SDF-1alpha+AMD3100 treatment
(AMD3100
200 ng/ml S+A group) |
NMSC+S+A
S0MSC+S+A
S1dMSC+S+A
S4dMSC+S+A
S7dMSC+S+A
S2wMSC+S+A
S1mMSC+S+A |
6.24±0.54
6.66±0.35
6.24±0.19
6.37±0.48
6.69±0.71
7.05±0.63
7.15±0.27 | | injured VSMCs (P group) |
NMSC+P1d
S0MSC+P1d
S1dMSC+P1d
S4dMSC+P1d
S7dMSC+P1d
S2wMSC+P1d
S1mMSC+P1d |
12.94±0.56*
30.69±0.47**##
34.46±0.73**##
32.58±0.61**##
34.82±0.39**##
33.73±0.54**##
33.15±0.81**## | | injured VSMCc+AMD3100 treatment
(P+A group) |
NMSC+P1d+A
S0MSC+P1d+A
S1dMSC+P1d+A
S4dMSC+P1d+A
S7dMSC+P1d+A
S2wMSC+P1d+A
S1mMSC+P1d+A |
10.56±0.11*
11.25±0.51*
11.68±0.48*
10.96±0.39*
11.23±0.16*
10.33±0.53*
10.98±0.72* | | injured VSMCs transfected with
SDF-1alpha siRNA (P+siRNA group) |
NMSC+P1d+siRNA
S0MSC+P1d+siRNA
S1dMSC+P1d+siRNA
S4dMSC+P1d+siRNA
S7dMSC+P1d+siRNA
S2wMSC+P1d+siRNA
S1mMSC+P1d+siRNA |
10.25±0.23*
10.54±0.67*
11.67±0.53*
10.98±0.28*
11.61±0.36*
10.88±0.17*
11.58±0.64* |
* statistically significant as compared with controls
** statistically significant vs NMSC group
# statistically significant vs normal BMSCs at the same group
statistically significant vs S+A group
SDF-1 can specifically bind to CXCR4 and then
induce the migration of monocytes, lymphocytes and
endothelial cells, which play important roles in the
embryonic development. In humans and rats, SDF-1
expression is sustained and can be regulated by the
changes in the surrounding environment. The SDF-1
gene is extremely conservative among different
species. Human SDF-1 gene is highly homologous
with murine SDF-1 gene (99%), and only one amino
acid was different between human SDF-1 gene and
murine SDF-1 gene. CXCR4, a receptor of SDF-1, is
coupled with a G protein, and an orphan receptor. The
CXCR4 gene is 32% homologous with the IL-8
receptor gene (Loetscher et al. 1994). CXCR4 is
constitutively expressed in a lot of cells, especially on
haematopoietic stem/progenitor cells. SDF-1 is the
unique ligand of CXCR4 which is the exclusive
receptor of SDF-1 having definite physiological
functions; the important difference between SDF-1/
CXCR4 and other chemokines/chemokine receptors.
When compared with other chemokines, SDF-1 has
more extensive biological activities and expressions
of SDF-1/CXCR4 vary from different systems
including the nervous system, the vascular system and
the haemopoietic system. Studies have shown that
SDF-1/CXCR4 play crucial biological roles in the
development of the haemopoietic system, nervous
system, the cardiovascular system and HIV infection
(Feng et al. 1996). SDF-1 can be constitutively
produced in many organs, but in bone marrow, SDF-1
is mainly expressed on endothelial cells and immature
osteoblasts. SDF-1 is a highly basic protein, and can
bind to the heparan sulfate on stromal cells through a
series of basic amino acid residuals. The signal area
in the N-terminal is then exposed and this facilitates
the binding between SDF-1 and CXCR4. The SDF-1
that binds to heparan sulfate is then fixed on the cell
membrane, which is the active form of SDF-1 (Amara
et al. 1999). The binding between SDF-1 and CXCR4
is the basis of the biological functions of SDF-1.
SDF-1 can confer potent chemotactic effects on
the in vitro CD34+ BMSCs in a dose dependent
manner (Yamaguchi et al. 2003). After intravenous
injection of adenovirus carrying SDF-1alpha plasmids,
HSCs were significantly motivated (Hattori et al.
2000, Moore et al. 2001), which however could be
suppressed by the neutralizing antibodies of CXCR4
and SDF-1 (Petit et al. 2002). After transfection with
the lentivirus carrying CXCR4 gene, CD34+ BMSCs
had an over-expression of CXCR4, which could
improve the chemotaxis induced by low dose SDF-1,
and prolong the survival time of progenitor cells with
CXCR4 over-expression. In addition, the
SDF-1/CXCR4 axis plays an important role in the
chemotaxis of bone marrow-derived cardiac
progenitor cells (Suda et al. 1987) in acute myocardial
infarction. Therefore, the SDF-1/CXCR4 axis is an
important participant in the motivation of bone
marrow stem cells and in the direction of
stem/progenitor cells to tissues.
In the present study, a Transwell chamber assay
was performed to evaluate the effects of SDF-1alpha and
injured VSMCs on the chemotaxis of BMSCs.
Results showed that the chemotaxis of normal
BMSCs was almost undetectable without SDF-1alpha
treatment. After SDF-1alpha treatment, the chemotaxis of
normal BMSCs was remarkably enhanced.
Furthermore, the chemotaxis of BMSCs from rats
with artery injury was more evident than that of
normal BMSCs after SDF-1alpha treatment. After
treatment with AMD3100, the chemotaxis of BMSCs
from rats with artery injury and normal BMSCs was
alleviated significantly. In addition, the normal
BMSCs and BMSCs from rats with artery injury were
co-cultured with injured VSMCs, and the results
showed that the chemotaxis of BMSCs was enhanced,
and more obvious than that of BMSCs induced by
SDF-1alpha alone. However, when injured VSMCs were
transfected with SDF-1alpha siRNA, the chemotaxis of
normal BMSCs and BMSCs BMSCs from rats with
artery injury was attenuated, but still more evident
than that of BMSCs induced by SDF-1alpha alone. After
treatment with AMD3100 to block the interaction
between SDF-1alpha and CXCR4, the chemotaxis of
BMSCs induced by injured VSMCs was alleviated.
These findings suggest that the interaction between
SDF-1alpha and CXCR4 was involved in the chemotaxis
of BMSCs induced by the injured VSMCs.
Moreover, the mRNA and protein expressions of
CXCR4 were determined in BMSCs. Results showed
the mRNA expression of CXCR4 gradually increased
and sustained after common carotid artery injury. This
result suggested the proportion of CXCR4+ cells was
increased after the BMSCs were stimulated, which
may be as a result of release of a variety of cytokines
and inflammatory factors. At the early stage of injury,
apoptosis and necrosis may induce the chemotaxis of
numerous inflammatory cells including neutrophils
and macrophages. A large number of cytokines and
growth factors are then released from inflammatory
cells, induce the migration of circulation TCSCs to
injured sites and are involved in the repair. In the
angiogenesis after injury, endothelial cells can
originate in two ways: (1) the adjacent vascular
endothelial cells can form new vessels through
sprouting or migration (Folkman and Shing 1992);
(2) the endothelial progenitor cells (EPCs), a
subpopulation of BMSCs, can form new vessels
through transdifferentiation (Shi et al. 1998). Thus,
BMSCs play an important role in angiogenesis.
Evidence has demonstrated that BMSCs could
aggregate in the post-ischemic hind legs of mice,
where angiogenesis has occurred, and could promote
angiogenesis (Asahara et al. 1997, Takahashi et al.
1999). In the migration of BMSCs to injured sites,
cytokines play a critical role. Numerous factors are
involved in the whole process of repair including
stem cell motivation related factors (G-CSF,
GM-CSF, SCF (Metcalf and Nicola 1983, Anderson
et al. 1990), SDF-1, IL-8 (Ulich et al. 1991, Laterveer
et al. 1995, Fibbe et al. 2000) and VEGF (Asahara et
al. 1999, Hattori et al. 2001, Paul and Steven 2002,
Simons and Ware 2003). In the present study, the
effect of SDF-1alpha on the chemotaxis of BMSCs was
detected through a Transwell chamber assay, and the
results demonstrated that SDF-1 could induce the
chemotaxis of BMSCs which could be blocked by
AMD3100, an antagonist of CXCR4. These findings
suggest that SDF-1 could induce the chemotaxis of
CXCR4+ cells among BMSCs. After injury, SDF-1alpha
expression increases gradually and a concentration
gradient of SDF-1alpha forms accompanied by increased
CXCR4 expression on cells. These changes may
promote migration of BMSCs to the injured sites and
their involvement in repair.
RNA interference (RNAi) is a potent tool. siRNA
is synthesized in vitro, and can specifically bind to a
target gene resulting in the silence of the target gene
and the suppressed expression of the target gene. In
the present study, SDF-1 siRNA was synthesized
and transfected into injured VSMCs. The results
showed that the mRNA and protein expressions of
SDF-1alpha were markedly decreased in VSMCs after
transfection with SDF-1alpha siRNA accompanied by
suppressed migration of BMSCs induced by injured
VSMCs, which may finally decrease the repair of
injured intima and result in subsequent intimal
thickening.
In the present study, VSMCs and BMSCs were
collected from normal rats and rats with common
carotid artery injury; the protein and mRNA
expression of SDF-1alpha and CXCR4 were determined.
In addition, the effects of VSMCs, SDF-1 siRNA
and AMD3100 on the chemotaxis of BMSCs were
investigated. The results showed that SDF-1alpha siRNA
could decrease the mRNA and protein expression of
SDF-1alpha, and AMD3100 treatment could inhibit the
chemotaxis of BMSCs induced by SDF-1alpha or injured
VSMCs. Additionally, the SDF-1alpha expression
increased in VMSCs after common carotid artery
injury and SDF-1alpha could induce the chemotaxis of
BMSCs. Based on these findings, we speculated that
increased SDF-1alpha expression in VMSCs after
common carotid artery injury could promote the
migration of BMSCs to injured sites where they are
involved in the repair of injury. Therefore, increasing
the circulating SDF-1alpha level at the early stage of
injury may recruit the CD34+CXCR4+ cells and
facilitate the endothelialisation of the injured artery
leading to construction of an integrated normal
endothelial barrier which can block the action of
circulating cytokines on the injured sites.
Additionally, at a late stage of injury, blocking the
interaction between SDF-1alpha and CXCR4 may prevent
the CD34+CXCR4+ cells from accumulating in the
injured sites and inhibit the post-traumatic
hyperplasia of vascular smooth muscle cells which is
also beneficial for the proliferation of newly
generated intimal cells.
REFERENCES
Amara A, Lorthioir O, Valenzuela A, Magerus A, Thelen M, Montes M, Virelizier JL, Delepierre M, Baleux F, Lortat-Jacob H, Arenzana-Seisdedos F. Stromal cell-derived factor-1 associates with heparin sulfates through the first beta-strand of the chemokine. J Biol Chem. 274: 23916-23925, 1999.
[CrossRef]
Anderson DM, Lyman SD, Baird A, Wignall JM, Eisenman J, Rauch C, March CJ, Boswell HS, Gimpel SD, Cosman D. Molecular cloning of mast cell growth factor, a hematopoietin that is active in both membrane bound and soluble forms. Cell. 63: 235-243, 1990.
[CrossRef]
Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 275: 964-966, 1997.
[CrossRef]
Asahara T, Takahashi T, Masuda H, Kalka C, Chen D, Iwaguro H, Inai Y, Silver M, Isner JM. VEGF contributes to postnatal neovascularization by mobilizing bone marrow-derived endothelial progenitor cells. EMBO J. 18: 3964-3972, 1999.
[CrossRef]
Barry FP, Murphy JM. Mesenchymal stem cells: clinical applications and biological characterization. Int J Biochem Cell Biol. 36: 568-584, 2004.
[CrossRef]
Bochaton-Piallat ML, Ropraz P, Gabbiani F, Gabbiani G. Phenotypic heterogeneity of rat arterial smooth muscle cell clones. Implications for the development of experimental intimal thickening. Arterioscler Thromb Vasc Biol. 16: 815-820, 1996.
[CrossRef]
Dowell JD, Rubart M, Pasumarthi KB, Soonpaa MH, Field LJ. Myocyte and myogenic stem cell transplantation in the heart. Cardiovasc Res. 58: 336-350, 2003.
[CrossRef]
Feng Y, Broder CC, Kennedy PE. HIV-1 entry co-factor: functional cDNA cloning of a seven-transmemberane, G protein-coupled receptor. Science. 272: 872, 1996.
[CrossRef]
Fibbe WE, Pruijt JF, von Kooyk Y. The role of metalloproteinases and adhesion molecules in interleukine-8-induced stem-cell mobilization. Semin Hematol. 37: 19-24, 2000.
[CrossRef]
Folkman J, Shing Y. Angiogenesis. J Biol Chem. 267: 10931-10934, 1992.
Gao C, Li Y. SDF-1 plays a key role in the repairing and remodeling process on rat allo-orthotopic abdominal aorta grafts. Transplant Proc. 39: 268-272, 2007.
[CrossRef]
Gleichmann M, Gillen C, Czardybon M, Bosse F, Greiner-Petter R, Auer J, Muller HW. Cloning and characterization of SDF-1gamma, a novel SDF-1 chemokine transcript with developmentally regulated expression in the nervous system. Eur J Neurosci. 12: 1857-1866, 2000.
[CrossRef]
Hattori K, Heissig B, Tashiro K, Honjo T, Tateno M, Shieh JH, Hackett NR, Quitoriano MS, Crystal RG, Rafii S, Moore MA. Plasma elevation of stromal cell-derived factor-1 induces mobilization of mature and immature hematopoietic progenitor and stem cells. Blood. 97: 3354-3360, 2000.
[CrossRef]
Hattori K, Dias S, Heissig B, Hackett NR, Lyden D, Tateno M, Hicklin DJ, Zhu Z, Witte L, Crystal RG, Moore MA, Rafii S. Vascular endothelial growth factor and angiopoitin-1 stimulate postnatal hematopoiesis by recruitment of vasculogenic and hematopoietic stem cells. J Exp Med. 193: 1005-1014, 2001.
[CrossRef]
Kucia M, Dawn B, Hunt G, Guo Y, Wysoczynski M, Majka M, Ratajczak J, Rezzoug F, Ildstad ST, Bolli R, Ratajczak MZ. Cells expressing early cardiac markers reside in the bone marrow and are mobilized into the peripheral blood after myocardial infarction. Circ Res. 95: 1191-1199, 2004a.
[CrossRef]
Kucia M, Jankowski K, Reca R, Wysoczynski M, Bandura L, Allendorf DJ, Zhang J, Ratajczak J, Ratajczak MZ. CXCR4-SDF-1 signaling, locomotion, chemotaxis and adhesion. J Mol Histol. 35: 233-245, 2004b.
[CrossRef]
Lapidot T, Petit I. Current understanding of stem cell mobilization: the roles of chemokines, proteolytic enzymes, adhesion molecules, cytokines, and stromal cells. Exp Hematol. 30: 973, 2002.
[CrossRef]
Laterveer L, Lindley IJ, Hamilton MS, Willemze R, Fibbe WE. Interleukin-8 induces rapid mobilization of hematopoietic stem cells with radioprotective capacity and long-term myelolymphoid repopulating ability. Blood. 85: 2269-2275, 1995.
Law RE, Meehan WP, Xi XP, Graf K, Wuthrich DA, Coats W, Faxon D, Hsueh WA. Troglitazone inhibits vascular smooth muscle cell growth and intimal hyperplasia. J Clin Invest. 98: 1897-1905, 1996.
[CrossRef]
Loetscher M, Geiser T, O’Reilly T, Zwahlen R, Baggiolini M, Moser B. Cloning of a human seven-transmembrane domain receptor, LESTR, that is highly expressed in leukocytes. J Biol Chem. 269: 232, 1994.
Mathur A, Martin JF. Stem cells and repair of the heart. Lancet. 364: 183-192, 2004.
[CrossRef]
Metcalf D, Nicola NA. Proliferative effects of purified granulocyte colony-stimulating factor, G-CSF on normal mouse hemopoietic cells. J Cell Physiol. 16: 198-206, 1983.
[CrossRef]
Minguell JJ, Erices A, Conget P. Mesechymal stem cells. Exp Biol Med. 226, 6: 507-552, 2001.
Misao Y, Takemura G, Arai M, Ohno T, Onogi H, Takahashi T, Minatoguchi S, Fujiwara T, Fujiwara H. Importance of recruitment of bone marrow-derived CXCR4+ cells in post-infarct cardiac repair mediated by G-CSF. Cardiovasc Res. 71: 455-465, 2006.
[CrossRef]
Moore MA, Hattori K, Heissig B, Shieh JH, Dias S, Crystal RG, Rafii S. Mobilization of endothelial and hematopoietic stem and progenitor cells by adenovector-mediated elevation of serum levels of SDF-1, VEGF, and angiopoietin-1. Ann NY Acad Sci. 938: 36-45, 2001.
[CrossRef]
Orlic D, Hill JM, Arai AE. Stem cells for myocardial regeneration. Circ Res. 91: 1092-1102, 2002.
[CrossRef]
Papayannopoulou T. Mechanisms of stem-/progenitor-cell mobilization: The anti-VLA-4 paradigm. Semin Hematol. 37 (Suppl. 2): 11-18, 2000.
[CrossRef]
Paul S, Steven N. Understanding coronary artery disease: Tomographic imaging with intravascular ultrasound. Heart. 88: 91-96, 2002.
[CrossRef]
Petit I, Szyper-Kravitz M, Nagler A. G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1and up-regulating CXCR4. Nat Immunol. 3: 687-694, 2002.
[CrossRef]
Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 284, 5411: 143-147, 1999.
[CrossRef]
Ratajczak MZ, Kucia M, Reca R, Majka M, Janowska-Wieczorek A, Ratajczak J. Stem cell plasticity revisited: CXCR4-positive cells expressing mRNA for early muscle. liver and neural cells ‘hide out’ in the bone marrow. Leukemia. 18: 29-40, 2004.
[CrossRef]
Ross R. The smooth muscle cell II. Growth of smooth cells in culture and formation of elastic fibers. J Cell Biol. 50: 172-176, 1971.
[CrossRef]
Schwartz SM. Biology of the neointima. Exp Nephrol. 2: 63-67, 1994.
Shi Q, Rafii S, Wu MH, Wijelath ES, Yu C, Ishida A, Fujita Y, Kothari S, Mohle R, Sauvage LR, Moore MA, Storb RF, Hammond WP. Evidence for circulating bone marrow-derived endothelial cells. Blood. 92: 362-367, 1998.
Simons M, Ware JA. Therapeutic angiogenesis in cardiovascular disease. Nat Rev Drug Discov. 2: 863-871, 2003.
[CrossRef]
Suda T, Suda J, Kajigaya S, Nagata S, Asano S, Saito M, Miura Y. Effects of recombinant murine granulocyte colony-stimulating factor on granulocyte-macrophage and blast colony formation. Exp Hematol. 15: 958-965, 1987.
Takahashi T, Kalka C, Masuda H, Chen D, Silver M, Kearney M, Magner M, Isner JM, Asahara T. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med. 5: 434-438, 1999.
[CrossRef]
Tulis DA, Durante W, Peyton KJ, Evans AJ, Schafer AI. Heme oxygenase-1 attenuates vascular remodeling following balloon injury in rat carotid arteries. Atherosclerosis. 155: 113-122, 2001.
[CrossRef]
Ulich TR, del Castillo J, Yi ES, Yin S, McNiece I, Yung YP, Zsebo KM. Hematologic effects of stem cell factor in vivo and in vitro in rodents. Blood. 78: 645-650, 1991.
Yamaguchi J, Kusano KF, Masuo O, Kawamoto A, Silver M, Murasawa S, Bosch-Marce M, Masuda H, Losordo DW, Isner JM, Asahara T. Stromal cell-derived factor-1 effects on ex vivo expanded endothelial progenitor cell recruitment for ischemic neovascularization. Circulation. 107: 1322-1328, 2003.
[CrossRef]
Zhang Y, Wang C, Yang Q. Establishing an organic model of SMC proliferation with cultured aorta of rats and exploring the underlying mechanism (in Chinese). Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 25: 1405-1410, 2008.
|
BACK
|





