Journal of APPLIED BIOMEDICINE
ISSN 1214-0287 (on-line)
ISSN 1214-021X (printed)
Volume 9 (2011), No 4, p 231-241
DOI 10.2478/v10136-011-0012-5
Roles of fibrin deposition and protease activated receptor-1 in renal cytokine/chemokine production and inflammatory cell infiltration in rats of different ages
Shupeng Lin, Xuefeng Sun, Suozhu Shi, Chunsheng Xi, Quan Hong, Yang Lu, Xiangmei Chen
Address: Xiangmei Chen, State Key Lab of Kidney Diseases, Chinese PLA General Hospital and Military Medical Postgraduate College, 28 Fuxing Road, Beijing 100853, China
xmchen301@126.com
Received 8th May 2011.
Revised 14th June 2011.
Published online 7th July 2011.
Full text article (pdf)
Summary
Key words
Introduction
Materials and methods
Results
Discussion
Acknowledgements
References
SUMMARY
The present study aimed to investigate the roles of fibrin deposition and protease activated receptor-1 (PAR-1) in renal cytokine/chemokine production and inflammatory cell infiltration in rats of different ages. Acute inflammation was induced by lipopolysaccharide (LPS) in rats which were then treated with tranexamic acid (TA), TA+urokinase (UK) or TA+low-molecular-weight heparin (HP). Fibrin deposition, inflammatory cells and expressions of PAR-1, monocyte chemoattractant protein-1 (MCP-1) and intercellular adhesion molecule 1 (ICAM-1) were detected. A reduction in fibrin deposition and PAR-1 expression in the LPS+TA+HP group was associated with decreased infiltration of inflammatory cells and down-regulated expressions of MCP-1 and ICAM-1. In the LPS+TA+UK group, the fibrin deposition, but not the PAR-1 expression, was reduced, However, the infiltration of inflammatory cells decreased and the expressions of MCP-1 and ICAM-1 down-regulated. There were significant differences in the fibrin deposition, infiltration of inflammatory cells and expression of PAR-1, MCP-1 and ICAM-1 between young and old rats undergoing the same treatment. These findings demonstrated that fibrin deposition plays more important roles than PAR-1 dose in cytokine/chemokine production and inflammatory cell infiltration in vivo, and ageing may deteriorate the fibrin deposition-induced production of cytokines/chemokines and infiltration of inflammatory cells.
KEY WORDS
coagulation; fibrin deposition; protease activated receptor-1; ageing; kidney
INTRODUCTION
Studies of acute and chronic inflammatory diseases in
humans have shown that a local increase in
procoagulants and fibrin deposition is important in the
pathogenesis of inflammatory injury (Furie and Furie
1988, Neale et al. 1988, Levi and van der Poll 2010).
This has also been confirmed in a variety of
glomerulonephritis (Neale et al. 1988, Cunningham et
al. 2004, Hertig and Rondeau 2004). Recent studies
reveal a close association between the coagulation
system and inflammation and immune responses.
Interestingly, it has been shown that blocking the
coagulation in the experimental glomerulitis can
attenuate the glomerular injury and local
inflammation, which indicates a pivotal cross-talk
between the coagulation and local inflammation
(Welty-Wolf et al. 2001, Miller et al. 2002).
Epidemiology shows an association between the
hypercoagulation state and the increased incidence of
thromboembolism in the elderly, which suggests
age-related changes in the vascular and hemostatic
systems. For example, some risk factors for
thromboembolism including fibrinogen, factor VII and
factor VIII were increased in the plasma of subjects
who are 60 years (Balleisen et al. 1985, Aillaud et al.
1986). The fibrin deposition in the glomerule was
reported to increase in lipopolysaccharide
(LPS)-treated aged mice (Yamamoto et al. 2002). In
addition, elderly individuals are more susceptible to
inflammatory stimuli than the young, and aged rats
demonstrate more serious inflammatory injury and an
increased mortality following endotoxin administration
as compared to young rats (Carthew et al. 1991).
However, whether an ageing-related hypercoagulation
state enhances the susceptibility to inflammatory
stimuli in elderly animals is as yet unclear.
Recent studies suggest that thrombin is a
physiological mediator of inflammatory events.
Administration of recombinant hirudin, a highly
specific thrombin antagonist, reduces the pathology
and leukocyte infiltration in a mouse glomerulo-nephritis model (Cunningham et al. 2000). Hirudin and
its analogs also prevent the occurrence of
inflammation and ameliorate the inflammation in the
carrageenin induced inflammation model (Cirino et al.
1996) and in mouse arthritis models (Varisco et al.
2000, Marty et al. 2001). These findings strongly
suggest that thrombin plays an important role in the
immunity and inflammation. Thrombin is a serine
protease that cleaves fibrinogen to form fibrin
monomers and uniquely cleaves cell surface receptors,
known as PARs. Evidence has demonstrated that
thrombin plays critical roles in the regulation of
inflammation in two ways: the biological activities of
fibrin and thrombin induced activation of the protease
activated receptor-1 (PAR-1).
PAR-1 is constitutively expressed in the glomerular
endothelial cells, mesangial cells and epithelial cells
and in the endothelial cells of the interstitial renal
vasculature. The in vitro PAR-1 activation by thrombin
or TRAP (a thrombin receptor activating peptide)
results in the production of pro-inflammatory
mediators, including IL-8 (Ueno et al. 1996),
E-selectin, platelet-derived growth factor (Shankar et
al. 1994), intercellular adhesion molecule-1 (ICAM-1)
and monocyte chemoattractant protein-1 (MCP-1)
(Grandaliano et al. 1994).
Thrombin may also influence inflammation
through stimulating the fibrin deposition. Fibrin is a
ligand for ICAM-1 (Languino et al. 1993),
CD11b/CD18 (CR3, Mac-1) (Diamond and Springer
1993) and CD11c/CD18 (CR4, p150/95) (Nham 1999).
Thus, extravascular fibrin may act as a provisional
adhesion matrix for leukocyte accumulation at sites of
inflammation. Moreover, fibrin can disrupt the
organization of endothelial cells and increase the
vascular permeability (Dang et al. 1985). In vitro
studies confirm that fibrin directly up-regulates the
expressions of ICAM-1 and MCP-1 in the endothelial
cells, and tumour necrosis factor alpha (TNF-alpha) and
IL-1beta expressions in the macrophages (Perez and
Roman 1995).
Until recently, few studies have evaluated the
effects of fibrin and PAR-1 on inflammation in the
elderly. In the present study, LPS was used to induce
renal acute inflammation in young and aged rats
which were then treated with tranexamic acid (TA)
alone, TA+urokinase (UK) or TA+ low-
molecular-weight heparin (HP). The expressions of
PAR-1 and fibrin and the inflammatory cell
infiltration were detected and the changes in the
mRNA and protein expressions of MCP-1 and
ICAM-1 investigated.
MATERIALS AND METHODS
Animals and Study Design
Young (3-month-old) and aged (28-month-old)
female Wistar rats, weighting 220±20 g and
420±30 g, respectively, were purchased from Beijing
Experimental Animal Centre and acclimatized in our
laboratory vivarium for 7 days before drug
administration. All animal procedures were performed
according to the Proper Care and Use of Laboratory
Animals. The animals were given ad libitum access to
food and water. Both young and aged rats were
randomly divided into five groups: normal control
group (NC group, n=8): rats were treated with saline
alone (vehicle for LPS); LPS group (n=8): rats were
intraperitoneally treated with LPS (14 mg/kg);
LPS+TA group (n=8): rats were treated with TA
(75 mg/kg) intraperitoneally at 30 min after LPS
administration; LPS+TA+HP group (n=8): rats were
subcutaneously treated with low-molecular-weight
heparin (200 U/kg) at 30 min before LPS
administration, and then intraperitoneally with TA
(75 mg/kg) at 30 min after LPS administration, and
the LPS+TA+UK group (n=8): rats were
intraperitoneally treated with TA (75 mg/kg) at
30 min after LPS administration and then
intravenously with UK (25,000 U/kg) 30 min later. At
4 h after LPS injection, the rats were sacrificed by an
overdose of inhalation anesthesia with ether. Kidney
tissues were collected. A fraction of the tissues was
immediately frozen in liquid nitrogen for isolation of
total RNA and protein extraction, and the remaining
tissues were embedded immediately in Optimal
Cutting Temperature (OCT) Compound (Miles
Scientific, Naperville, USA) and then snap-frozen in
liquid nitrogen for immunofluorenscence staining as
described below.
Reagents
LPS was purchased from Sigma Chemical Co. (St
Louis, USA) and dissolved in 0.9% saline before use.
TA Injection and UK Injection were purchased from
Dongting Pharmaceuticals Co., Ltd. (Hunan Province,
China) and Fengyuan Pharmaceutical Factory (Anhui
Province, China), respectively.
The following monoclonal and polyclonal
antibodies were used in this study: mouse anti-rat
CD11b (Mac-1 alpha-chain) and WT.5 (BD Pharmigen)
which label neutrophils and some myeloid cells;
FITC-conjugated fibrin polyclonal antibody (Dako
Ltd., Glostrup, Denmark); mouse anti-rat ICAM-1 and
PAR-1 monoclonal antibody and goat anti-rat MCP-1
polyclonal antibody were purchased from Santa Cruz
(Santa Cruz Biotechnology Inc., Santa Cruz, USA).
Peroxidase-conjugated anti-mouse/goat IgG was
purchased from Beijing Zhongshan Golden Bridge
Biotechnology Co. Ltd. (Beijing, China).
Immunofluorescence staining for fibrin and CD11b
Tissue sections frozen in OCT (Miles Laboratories,
Elkhart, USA) were cut into serial sections (4 m in
thickness) on a cryostat, and fixed in acetone for 5 min
at room temperature. The detection of fibrin was
performed using a direct method, with a
FITC-conjugated rabbit anti-fibrin antibody.
Fluorescent images were obtained with a confocal
laser scanning microscope (Bio-Rad MRC1024ES,
Bio-Rad Laboratories Inc., Hercules, USA). Fibrin
deposition at a minimum of 30 glomeruli per rat was
quantitatively evaluated by measuring the intensity of
fluorescence in the glomerular areas with LaserPix 4.0
software (Bio-Rad Laboratories Inc., Hercules, USA).
For the evaluation of infiltrating neutrophils, CD11b
immunofluorescence staining was performed with an
indirect method. A minimum of 20 glomeruli was
assessed per animal. and results were expressed as cell
number per glomerular cross section (c/gcs).
Northern blot for MCP-1, ICAM-1 and PAR-1
Total RNA was extracted from the kidney tissues
using TRIzol (GIBCO BRL, Grand Island, USA)
according to the manufacturer's directions. RNA
(20 g) was denatured and electrophoresed through a
1% agarose gel containing formaldehyde and
transferred to Hybond® N+ nylon membranes
(Amersham Biosciences, UK) by capillary action. The
transferred RNAs were cross-linked to the nylon
membrane with an ultraviolet light cross linker. The
quality of RNA was assessed by ethidium bromide
staining. After transferring, the blots were
pre-hybridized at 42 °C for 3 h. Then, membranes
were hybridized with each cDNA probe labelled by
the random primer method (Boehringer Mannheim
Biochemica, Germany) with [alpha-32P]-dCTP at 42 °C
for 20 h. After hybridization, the blots were washed
twice with 2× standard saline citrate (SSC), 0.1%
sodium dodecyl sulfate (SDS), and then once with
0.1 × SSC/0.1% SDS at 42 °C for 15 min. The
hybridized membranes were exposed at -70 °C for
72 h. Autoradiography films (Kodak, Rochester,
USA) were scanned using the UVP-2000 system. For
quantitative densitometric measurements of Northern
blots, all the signals were normalized by comparison
with the signals of 28S RNA.
The primers for MCP-1 were as follows: sense,
5´-ATG CAG GTC TCT GTC ACG-3´, antisense,
5´-CTA GTT CTC TGT CAT ACT-3´(94 °C for 30 s,
55 °C for 30 s, 72 °C for 60 s, 28 cycles, product size:
448 bp). The primers for ICAM-1 were: sense,
5´-GAT GCT GAC CCT GGA GAG CA-3´,
antisense, 5´-CAG GGA CTT CCC ATC CAC CT-3´
(94 °C for 45 s, 55 °C for 30 s, 72 °C for 90 s, 35
cycles, product size: 409 bp). The primers for PAR-1
were: sense, 5´-CCGCAGCGTGTTATT-3´,
antisense, 5´-CGCAGAGGAGGTAAG-3´ (94 °C for
30 s, 58 °C for 30 s, 72 °C for 30 s, 35 cycles, product
size: 389 bp).
Western blot for MCP-1, PAR-1 and ICAM-1
Tissues were homogenized in 1 ml of lysis buffer
(20 mM HEPES-KOH, 250 mM sucrose, 10 mM
KCl, 1.5 mM MgCl2, 1 mM EDTA, 1 mM EGTA, 1
mM dithiothreitol, digitonin at 500 l/ml, 0.1 mM
phenylmethylsulphonylfluoride, aprotinin at 2 mg/ml,
leupeptin at 10 mg/ml, and pepstatin at 5 mg/ml),
with a handheld homogenizer. All samples were
centrifuged at 10,000 g for 30 min at 4 °C, and the
protein concentration in each lysate was determined
spectrophotometrically. The proteins extracted were
solubilized by boiling in SDS loading buffer. Then,
50 microg of total protein were separated by 10%
SDS-polyacrylamide gel electrophoresis and
transferred onto a 0.45 microm-pore nitrocellulose
membrane (Schleicher & Schuell, Dassel, Germany)
by the semidry method (Bio-Rad Laboratories, Hercules, USA). The nitrocellulose membranes were
blocked in 10 ml of Tris-buffered saline (TBS) buffer
(10 mM Tris-HCl, 0.15 M NaCl, 8 mM sodium azide,
0.05% Tween-20) containing 3% bovine serum
albumin (BSA) overnight at 4 °C. The membranes
were then incubated with the primary antibody, goat
anti-rat MCP-1 polyclonal antibody, mouse anti-rat
ICAM-1 and PAR-1 monoclonal antibody (1:200) for
2 h at room temperature. Thereafter, the nitrocellulose
membrane was washed three times with TBS
containing 3% BSA, and incubated for 90 min at room
temperature with peroxidase-conjugated AffiniPure
goat anti-mouse IgG (1:2000). After washing with
TBS, blots were developed with enhanced
chemiluminescent (ECL) reagents. Rabbit polyclonal
anti-beta-actin antibody (1:100, Santa Cruz Bio-technology) was used as the control for each sample.
Statistical analysis
Statistical analysis was performed using SPSS
software (SPSS, Inc., Chicago, USA). Quantitative
data were expressed as mean±standard deviation (SD)
and analysed by one-way analysis of variance
(ANOVA), followed by the LSD post hoc test, for
comparisons of the difference among the rats in five
groups of the same age group. Student's t-test or
analysis of covariance (ANCOVA) was used to
compare the difference between two age groups with
same treatment. We used the significance level
2alpha=0.05.
RESULTS
Induction and regulation of glomerular fibrin
deposition
Fibrin immunofluorenscence staining was evaluated
quantitatively by measuring the intensity of
fluorescence in the glomerular areas (Fig. 1). There
was almost no fibrin deposition in the glomeruli of
young and aged rats in the NC group. LPS could
induce the fibrin deposition in the young and aged rats
in the LPS group. In addition, LPS+TA increased the
fibrin deposition, while LPS+TA+HP and
LPS+TA+UK decreased it. The fibrin fluorescence
intensity in the aged rats was higher than that in the
young ones with the same treatment (statistically
significant). There was no significant difference in the
fibrin fluorescence intensity between LPS+TA+HP
group and LPS+TA+UK group.
Changes of PAR-1 protein and mRNA expressions
PAR-1 protein expressions and quantitative image
analysis are shown in Fig. 2A and B, respectively.
There was little PAR-1 expression in the glomeruli of
normal young rats. In the NC group, the protein
expression of PAR-1 in the aged rats was higher than
that in the young ones (0.52±0.11 vs. 0.25±0.03,
statistically significant). In the LPS group, the protein
expression of PAR-1 in the aged rats was higher than
that in the young animals (1.61±0.31 vs. 0.33±0.07,
statistically significant). In the LPS+TA group, the
protein expression of PAR-1 in young (0.42±0.10)
and aged (1.62±0.22) rats were slightly higher than
that in the LPS group, but without significant
difference. When compared with the LPS+TA group,
PAR-1 expression in young (0.20±0.06) and aged
(1.0±0.21) rats of LPS+TA+HP groups was markedly
decreased (statistically significant). However, PAR-1
expression in the young (0.38±0.13) and aged
(1.54±0.22) rats of LPS+TA+UK groups was not
markedly decreased when compared with that of
LPS+TA groups (statistically not significant).
The results from Northern blot are shown in Fig.
2C and D. Densitometric analysis revealed that the
changes in the PAR-1 mRNA expression were similar
to that of protein expression.
Infiltration of CD11b positive cells in glomeruli is
PAR-1 independent, but fibrin dependent
Most of the infiltrating cells were neutrophils in the
acute phase of LPS induced inflammation (Naruse et
al. 1985). The immunofluorescence staining of
CD11b, a marker of neutrophils, can reveal the source
of infiltrating cells, and was evaluated by positive cell
count (Fig. 3). There were few CD11b positive cells
in the glomeruli of young and aged rats of the NC
groups. The number of CD11b positive cells of the
aged rats in the LPS group was higher than that of the
young rats (13.5±3.4 vs. 7.9±2.1 c/gcs, statistically
significant). The number of CD11b positive cells was
significantly augmented in the glomeruli of the young
and the aged rats in the LPS+TA group when
compared with that in the LPS group, while the
number of CD11b positive cells of aged rats in the
LPS+TA group was significantly higher than that of
the young ones (21.5±5.3 vs. 14.8±3.6, statistically
significant). Compared with the LPS+TA group, the
number of CD11b positive cells was significantly
reduced in the glomeruli of young and aged animals
in the LPS+TA+HP group (7.2±0.8, vs. 17.5±3.9,
statistically significant). In the young and aged rats of
LPS+TA+UK group, the CD11b positive cells were
markedly reduced when compared with those in the
LPS+TA group (7.5±2.9, vs. 16.5±3.1, statistically
significant). The changes in the number of CD11b
positive cells were similar to those in the fibrin
deposition in the glomeruli but not in PAR-1
expression. In LPS+TA groups, the fibrin deposition
increased, while the expression of PAR-1 was not
changed as compared to LPS groups, and the CD11b
positive cells still increased. Similarly, in the
LPS+TA+UK groups, the fibrin deposition decreased,
but PAR-1 expression was similar to that in the
LPS+TA groups, and the CD11b positive cells also decreased. The findings indicated that CD11b
positive cell infiltration in the glomeruli is PAR-1
independent, but dependent on fibrin.
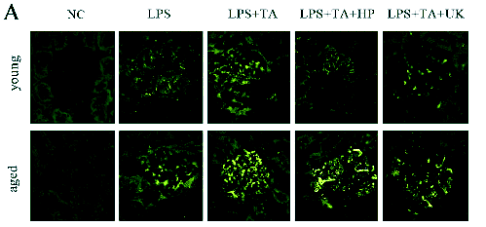
Fig. 1. Fibrin immunofluorescence staining. (A) Representative photomicrographs from confocal laser scanning. (×400). (B) Immunofluorescence density in the immunofluorescence staining of fibrin. Data were expressed as mean ± SD.
* statistically significant as compared with LPS+TA group with the same age
# statistically significant vs. young rats with the same treatment
Changes in the in vivo productions of MCP-1 and ICAM-1 were fibrin dependent, but PAR-1 independent
MCP-1 protein expressions and quantitative image
analysis are shown in Fig. 4A and B, respectively.
MCP-1 was hardly detectable in the normal glomeruli
of the young and the aged rats. The MCP-1
expression in the glomeruli of the aged rats was
higher than that of the young rats in the LPS group
(statistically significant). Moreover, the MCP-1
expression in the young and aged rats in the LPS+TA
group was markedly higher than that in the LPS
group, while in the aged rats of the LPS+TA group it
was significantly higher than that of the young ones
(statistically significant). When compared with the
LPS+TA group, the MCP-1 expression of both young
and aged rats in the LPS+TA+HP group was
markedly decreased, and higher in the aged rats of the
LPS+TA+HP group than in the young animals
(statistically significant). In the young and aged rats
of the LPS+TA+UK group, the MCP-1 expression
was significantly reduced as compared to the
LPS+TA group (statistically significant). Northern
blot for MCP-1 (Fig. 4C and D) revealed that the
changes in the MCP-1 mRNA expression were
similar to the protein expression of MCP-1.
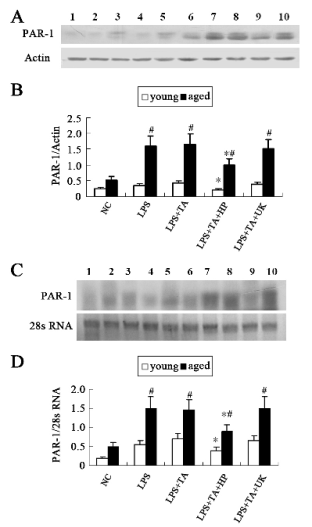
Fig. 2. Protein and mRNA expressions of PAR-1. (A) Western blot assay of PAR-1. (B) Densitometric analysis of bands in
Western blot. (C) Northern blot assay of PAR-1. (D) Densitometric analysis of bands in Northern blots. Data were expressed
as mean±SD. Line 1, 2, 3, 4 and 5 represent young NC group, LPS group, LPS+TA group , LPS+TA+HP group and
LPS+TA+UK group, respectively. Line 6, 7, 8, 9 and 10 represent aged NC group, LPS group, LPS+TA group,
LPS+TA+HP group and LPS+TA+UK group respectively.
* statistically significant as compared with LPS+TA group with the same age
# statistically significant vs. young rats with the same treatment
The ICAM-1 protein expressions and quantitative
image analysis are shown in Fig. 5A and B,
respectively. The protein expression of ICAM-1 in
the aged animals was higher than that in young
animals in the LPS group (1.03±0.31 vs. 0.21±0.08%,
statistically significant). Moreover, the ICAM-1
protein expression in young and aged rats in the
LPS+TA group was markedly higher than that in the
LPS group, and in the aged rats of the LPS+TA group
it was dramatically higher than in the young rats
(1.22±0.34 vs. 1.01±0.29%, statistically significant).
When compared with the LPS+TA group, the
ICAM-1 protein expression in the LPS+TA+HP and
LPS+TA+UK groups was markedly decreased, and in
the aged animals of the LPS+TA+HP and
LPS+TA+UK groups it was also higher than in the
young ones (0.77±0.24 vs. 1.21±0.31, 0.81±0.23
vs.1.31±0.34, respectively, statistically significant). Results of Northern blot are shown in Fig. 5C and D.
The changes in the ICAM-1 mRNA expression were
similar to those in the protein expression.

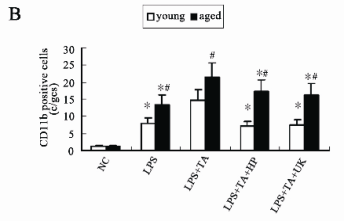
Fig. 3. CD11b immunofluorescence staining. (A) Representative photomicrographs from confocal laser scanning. (×400). (B) CD11b positive cells in the immunofluorescence staining. Data were expressed as mean ± SD.
* statistically significant as compared with LPS +TA group with the same age
# statistically significant vs. young rats with the same treatment
In the LPS+TA groups, the fibrin deposition
increased, while the PAR-1 expression was not
changed as compared to LPS groups, and the
expressions of MCP-1 and ICAM-1 still increased.
Similarly, in the LPS+TA+UK groups, the fibrin
deposition decreased, but the PAR-1 expression
remained relatively stable as compared to the
LPS+TA groups, and the expressions of MCP-1 and
ICAM-1 also decreased. The expressions of MCP-1
and ICAM-1 had a closer relationship with fibrin
deposition than PAR-1 did. These findings indicated
that fibrin but not PAR-1 may regulate the MCP-1
and ICAM-1 production in vivo.
DISCUSSION
Recent studies suggest that thrombin is an important
physiological mediator in inflammatory events in two
ways: the biological activities of fibrin, and the
thrombin induced activation of PAR-1. But the
contributions of PAR-1 and fibrin to the inflammation
in older subjects are still unknown.
In the present study, LPS was used to induce
inflammation in young and old rats which were thentreated with TA, HP and UK, with the aim of
investigating the in vivo pro-inflammatory effects of
fibrin and PAR-1. TA increased the fibrin deposition
through its antifibrinolytic effects, HP reduced the
fibrin deposition by binding anti-thrombin III, and
UK reduced the fibrin deposition through its profibrinolytic effects. Our data showed there were significant changes in the fibrin deposition and
expression of PAR-1 among five different treatment
groups, which demonstrated that TA, HP and UK
could interfere with the level of glomerular fibrin
deposition and expression of PAR-1. The animal
model and methods used in this study facilitated the
investigation of the pro-inflammatory effects of fibrin
and PAR-1 in vivo.
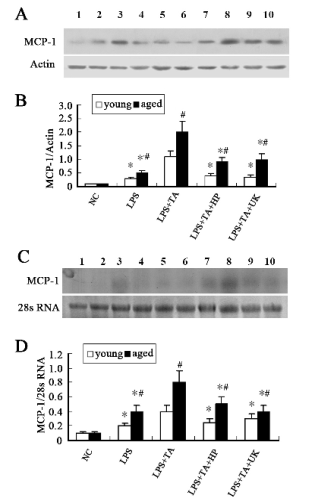
Fig. 4. Protein and mRNA expressions of MCP-1. (A) Western blot assay of MCP-1. (B) Densitometric analysis of bands in
Western blot. (C) Northern blot assay of MCP-1. (D) Densitometric analysis of bands in Northern blot. Data were expressed as
mean±SD. Line 1, 2, 3, 4 and 5 represent young NC group, LPS group, LPS+TA group, LPS+TA+HP group and LPS+TA+UK
group, respectively. Line 6, 7, 8, 9 and 10 represent aged NC group, LPS group, LPS+TA group, LPS+TA+HP group and
LPS+TA+UK group respectively.
* statistically significant as compared with LPS+TA group with the same age
# statistically significant vs. young rats with the same treatment
A critical characteristic of inflammatory disease is
the migration of leukocytes from the circulation
across the endothelium, and into the affected tissues.
Leukocyte extravasation from the blood into the
tissues is a multistep process involving a series of
coordinated interactions between leukocytes and
endothelial cells. MCP-1 and ICAM-1 are important
molecules involved in leukocyte extravasation. In the
glomerulus, MCP-1 is mainly detectable in the vascular endothelial cells. MCP-1 is predominantly
expressed on the surface of endothelial cells and can
interact with their cognate receptors on the specific
leukocytes, which triggers the activation of adhesion
molecules resulting in tight adhesion (Wada et al.
2001). ICAM-1 mediates the tight adhesion between
leukocytes and endothelial cells. It has been reported
that ICAM-1 can recognize the bridging ligand
fibrin(ogen) (Altieri 1999). To further explore the
pro-inflammatory effects of fibrin deposition and
PAR-1, we determined the changes in the protein and
mRNA expressions of MCP-1 and ICAM-1. Our
results showed that, in the LPS+TA groups, the fibrin
deposition increased, while the PAR-1 expression
was not changed as compared to the LPS groups, and
the expressions of MCP-1 and ICAM-1 still
increased. Similarly, in the LPS+TA+UK groups, the
fibrin deposition decreased, but the PAR-1
expressions remained relatively stable as compared to
the LPS+TA groups, and the expressions of MCP-1
and ICAM-1 correspondingly decreased. The findings
imply that it is fibrin but not PAR-1 that activates the
in vivo productions of chemokine/cytokines including
MCP-1 and ICAM-1, which then stimulate the
macrophage adhesion.
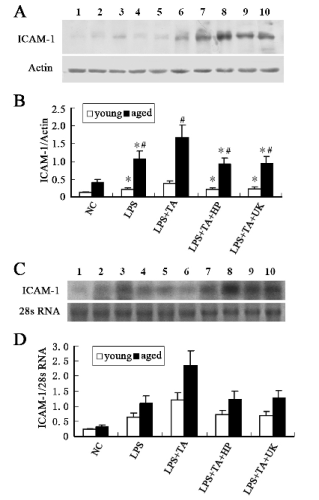
Fig. 5. Protein and mRNA expressions of ICAM-1. (A) Western blot assay of ICAM-1. (B) Densitometric analysis of bands
in Western blot. (C) Northern blot assay of ICAM-1. (D) Densitometric analysis of bands in Northern blot. Data were expressed
as mean±SD. Line 1, 2, 3, 4 and 5 represent young NC group, LPS group, LPS+TA group, LPS+TA+HP group and LPS+TA+UK
group, respectively. Line 6, 7, 8, 9 and 10 represent aged NC group, LPS group, LPS+TA group, LPS+TA+HP group and
LPS+TA+UK group respectively.
* statistically significant as compared with LPS+TA group with the same age
# statistically significant vs. young rats with the same treatment
Szaba and Smiley (2002) investigated roles for
thrombin, PAR-1, and fibrinogen in PAR-1 deficient
and fibrinogen-deficient mice with peritonitis. Their
results demonstrated that thrombin played an
important role in stimulating the in vivo adhesion of
inflammatory peritoneal macrophages, which is
PAR-1 independent, but dependent on fibrinogen.
They also revealed that thrombin could stimulate the
peritoneal accumulation of cytokines and chemokines
in a fibrinogen-dependent manner. These findings
were consistent with ours.
Of course, there is controversy on the
pro-inflammatory effects of fibrin and PAR-1. In the
study by Cunningham et al (2000), PAR-1 deficient
mice were used to establish a crescentic
glomerulonephritis model and results showed
significant protection from crescentic glomerulo-nephritis when compared with wild-type mice:
crescent formation, inflammatory cell infiltration and
serum creatinine significantly reduced in the PAR-1
deficient mice. The findings suggest that
receptor-mediated effects of thrombin rather than its
coagulant effects are responsible for the majority of
the contributions of thrombin to the renal injury in
this model. Of note is the fact that, although the
systemic coagulation, platelet count and function
were normal in PAR-1 deficient mice, these mice had
significantly less fibrin deposition in the glomeruli
during development of glomerulonephritis. For this
reason, the pro-inflammatory effects of fibrin
deposition can not be excluded.
Interestingly, in the young and aged rats receiving
the same treatment, there was a significant difference
in fibrin deposition between them, suggesting that
there was a difference in the response to the same
stimuli between young and aged rats. Taken together,
the aged rats were more susceptible to the
development of fibrin deposition, indicating that
ageing accelerates the glomerular fibrin deposition,
which was consistent with results in the study of
Yamamoto et al (2002). Our data on age differences
in rats suggest a similar picture in human subjects.
Clinical studies have shown that elderly individuals
are susceptible to endotoxin-induced effects than the
young, and with increased susceptibility to
haemorrhage and intravascular hypercoagulation
following endotoxin administration (Horan and
Pendleton 1995). In other studies on haematological
disease, aged mammals which are healthy are also
susceptible to experimental drug-induced marrow
hypoplasia or anaemia (Berger 1987a, b). In addition,
there was also a significant difference in the
expressions of MCP-1 and ICAM-1 between young
and aged rats, suggesting that more fibrin deposition
in aged rats facilitates the glomerular inflammatory
cell infiltration by up-regulating MCP-1 and ICAM-1
expressions.
In summary, our findings provide substantial
evidence that fibrin deposition plays more important
roles than PAR-1 dose in the cytokine/chemokine
production and inflammatory cell infiltration in vivo,
and that ageing may promote glomerular fibrin
deposition and subsequent inflammatory response.
Our results provide novel insights into the
associations among coagulation, inflammation and
ageing.
ACKNOWLEDGEMENTS
This work was supported by the Major State Basic
Research Development Program of China
(2007CB507400).
REFERENCES
Aillaud MF, Pignol F, Alessi MC, Harle JR, Escande M, Mongin M, Juhan-Vague I. Increase in plasma concentration of plasminogen activator inhibitor, fibrinogen, von Willebrand factor, factor VIII: C and in erythrocyte sedimentation rate with age. Thromb Haemost. 55: 330-332, 1986.
Altieri DC. Regulation of leukocyte-endothelium interaction by fibrinogen. Thromb Haemost. 82: 781-786, 1999.
Balleisen L, Bailey J, Epping PH, Schulte H, van de Loo J. Epidemiological study on factor VII, factor VIII and fibrinogen in an industrial population. I. Baseline data on the relation to age, gender, bodyweight, smoking, alcohol, pill-using, and menopause. Thromb Haemost. 54: 475-479, 1985.
Berger J. Age-related sensitivity of rats to induction of anaemia. Fol Haematol. 114: 408-413, 1987a.
Berger J. Age-associated sensitivity to experimental drug-induced marrow hypoplasia in laboratory rats. Haematologia. 20: 171-178, 1987b.
Carthew P, Dorman BM, Edwards RE. Increased susceptibility of aged rats to haemorrhage and intravascular hypercoagulation following endotoxin administered in a generalized Shwartzman regime. J Comp Pathol. 105: 323-330, 1991.
Cirino G, Cicala C, Bucci MR, Sorrentino L, Maraganore JM, Stone SR. Thrombin functions as an inflammatory mediator through activation of its receptor. J Exp Med. 183: 821-827, 1996.
Cunningham MA, Rondeau E, Chen X, Coughlin SR, Holdsworth SR, Tipping PG. Protease-activated receptor 1 mediates thrombin-dependent, cell-mediated renal inflammation in crescentic glomerulonephritis. J Exp Med. 191: 455-462, 2000.
Cunningham MA, Kitching AR, Tipping PG, Holdsworth SR. Fibrin independent proinflammatory effects of tissue factor in experimental crescentic glomerulonephritis. Kidney Int. 66: 647-654, 2004.
[CrossRef]
Dang CV, Bell WR, Kaiser D, Wong A. Disorganization of cultured vascular endothelial cell monolayers by fibrinogen fragment D. Science. 227: 1487-1490, 1985.
Diamond MS, Springer TA. A subpopulation of Mac-1 (CD11b/CD18) molecules mediates neutrophil adhesion to ICAM-1 and fibrinogen. J Cell Biol. 120: 545-556, 1993.
Furie B, Furie BC. The molecular basis of blood coagulation. Cell. 53: 505-518, 1988.
Grandaliano G, Valente AJ, Abboud HE. A novel biologic activity of thrombin: stimulation of monocyte chemotactic protein production. J Exp Med. 179: 1737-1741, 1994.
[CrossRef]
Hertig A, Rondeau E. Role of the coagulation/fibrinolysis system in fibrin-associated glomerular injury. J Am Soc Nephrol. 15: 844-853, 2004.
Horan MA, Pendleton N. The relationship between aging and disease. Clin Geron. 5: 125-141, 1995.
Languino LR, Plescia J, Duperray A, Brian AA, Plow EF, Geltosky JE, Altieri DC. Fibrinogen mediates leukocyte adhesion to vascular endothelium through an ICAM-1-dependent pathway. Cell. 73: 1423-1434, 1993.
Levi M, van der Poll T. Inflammation and coagulation. Crit Care Med. 38(Suppl. 2):S26-34, 2010.
[CrossRef]
Marty I, Peclat V, Kirdaite G, Salvi R, So A, Busso N. Amelioration of collagen-induced arthritis by thrombin inhibition. J Clin Invest. 107: 631-640, 2001.
[CrossRef]
Miller DL, Welty-Wolf K, Carraway MS, Ezban M, Ghio A, Suliman H, Piantadosi CA. Extrinsic coagulation blockade attenuates lung injury and proinflammatory cytokine release after intratracheal lipopolysaccharide. Am J Respir Cell Mol Biol. 26: 650-658, 2002.
Naruse T, Tsuchida A, Ogawa S, Yano S, Maekawa T. Selective glomerular thrombosis in rats induced by combined injections of nephrotoxic antiserum and lipopolysaccharide. J Lab Clin Med. 105: 146-156, 1985.
Neale TJ, Tipping PG, Carson SG, Holdsworth SR. Participation of cell-mediated immunity in deposition of fibrin in glomerulonephritis. Lancet. 2: 421-424, 1988.
Nham SU. Characteristics of fibrinogen binding to the domain of CD11c, an alpha subunit of p150,95. Biochem Biophys Res Commun. 264: 630-634, 1999.
[CrossRef]
Perez RL, Roman J. Fibrin enhances the expression of IL-1beta by human peripheral blood mononuclear cells: implications in pulmonary inflammation. J Immunol. 154: 1879-1887, 1995.
Shankar R, de la Motte CA, Poptic EJ, DiCorleto PE. Thrombin receptor-activating peptides differentially stimulate platelet-derived growth factor production, monocytic cell adhesion, and E-selectin expression in human umbilical vein endothelial cells. J Biol Chem. 269: 13936-13941, 1994.
Szaba FM, Smiley ST. Roles for thrombin and fibrin(ogen) in cytokine/chemokine production and macrophage adhesion in vivo. Blood. 99: 1053-1059, 2002.
[CrossRef]
Ueno A, Murakami K, Yamanouchi K, Watanabe M, Kondo T. Thrombin stimulates production of interleukin-8 in human umbilical vein endothelial cells. Immunology. 88: 76-81, 1996.
Varisco PA, Peclat V, van Ness K, Bischof-Delaloye A, So A, Busso N. Effect of thrombin inhibition on synovial inflammation in antigen induced arthritis. Ann Rheum Dis. 59: 781-787, 2000.
Wada T, Yokoyama H, Matsushima K, Kobayashi K. Chemokines in renal diseases. Int Immunopharmacol. 1: 637-645, 2001.
Welty-Wolf KE, Carraway MS, Miller DL, Ortel TL, Ezban M, Ghio AJ, Idell S, Piantadosi CA. Coagulation blockade prevents sepsis induced respiratory and renal failure in baboons. Am J Respir Crit Care Med. 164: 1988-1996, 2001.
Yamamoto K, Shimokawa T, Yi H, Isobe K, Kojima T, Loskutoff DJ, Saito H. Aging accelerates endotoxin -induced thrombosis increased responses of plasminogen activator inhibitor-1 and lipopolysaccharide signaling with aging. Am J Pathol. 161: 1805-1814, 2002.
|
BACK
|







