Journal of APPLIED BIOMEDICINE
ISSN 1214-0287 (on-line)
ISSN 1214-021X (printed)
Volume 9 (2011), No 4, p 197-207
DOI 10.2478/v10136-011-0006-3
Oral melatonin administration and programmed cell death of neutrophils, lymphocytes, and other cell types from rats injected with HL-60 cells
Jonathan Delgado, Maria del Pilar Terron, Virginio Garcia-Martinez, Carmen Lopez-Sanchez, Carmen Barriga, Jose Antonio Pariente, Ana Beatriz
Rodriguez
Address: Maria del Pilar Terron Sanchez, Department of Physiology, Faculty of Science, University of Extremadura, Avda. de Elvas s/n, 06006 Badajoz,
Spain
pilarts@unex.es
Received 23rd February 2011.
Revised 15th April 2011.
Published online 29th June 2011.
Full text article (pdf)
Summary
Key words
Introduction
Material and Methods
Results
Discussion
Acknowledgements
References
SUMMARY
Recent years have seen mounting evidence for the role of melatonin in mediating programmed cell death, with a protective, anti-apoptotic effect in
healthy cells, but an anti-tumoural, pro-apoptotic action in many tumour cells. In this study, we evaluated the effect of melatonin on the
programmed cell death induced by thapsigargin (TG), on lymphocytes and neutrophils, and on various tissues obtained from rats injected with human
promyelocytic leukaemia cells (HL-60), treated with melatonin in their drinking water (20 microm), and fed ad libitum. Melatonin treatment
significantly reduced caspase-3 and -9 activity, and caused the proportions of lymphocytes, neutrophils, and eosinophils to revert to their basal
values. No histological differences were observed. In conclusion, melatonin has anti-apoptotic effects on lymphocytes and neutrophils obtained from
rats injected with HL-60 leukaemia cells.
KEY WORDS
melatonin; neutrophils; lymphocytes; apoptosis; HL-60 cells
INTRODUCTION
Melatonin is a physiological mediator that is present
at all evolutionary levels, from bacteria to humans
(Reiter 1991, Hardeland and Fuhrberg 1996). In
mammals, melatonin is produced by the pineal gland
and by a variety of extrapineal tissues (Panke et al.
1979, Carrillo-Vico et al. 2005, Kobayashi et al.
2005). Among its actions in controlling seasonal
reproduction in photoperiodic mammals (Reiter and
Fraschini 1969, Reiter 1973), melatonin has a major
role in controlling tumour development and growth,
and its anti-proliferative activity has been
demonstrated both in vivo and in vitro in different cell
systems (Cos et al. 2001, 2006, Berger 2008,
Bejarano et al. 2009, Dauchy et al. 2009, Park et al.
2010).
Programmed cell death is a genetically
predetermined mechanism that can operate via two
molecular pathways - the extrinsic pathway and the
intrinsic pathway. In the extrinsic pathway (also
known as the "death receptor pathway"), programmed
cell death is caused by ligand-induced activation of
death receptors on the cell surface. Such death
receptors include receptor-1 of the tumour necrosis
factor (TNF), CD95/Fas (the CD95L/FasL receptor),
and receptors-1 and -2 of the TNF-related
programmed cell death-inducing ligand (TRAIL). In
the intrinsic pathway (also known as the
mitochondrial pathway), programmed cell death is the
result of a cascade of intracellular events in which
mitochondrial permeabilization plays a crucial role
(Scaffidi et al. 1998). The proteins of the Bcl-2 family
regulate programmed cell death by acting on the
mitochondria. Once activated, the pro-apoptotic
proteins of this family increase the permeability of the
inner mitochondrial membrane (mitochondrial
permeability transition pore, mPTP) and open a pore
in the outer mitochondrial membrane that allows the
release of numerous pro-apoptotic proteins from the
intermembrane space (Hajnoczyk et al. 2003),
including SMAC/DIABLO (which blocks caspase
inhibitors) and cytochrome c. Once in the cytosol,
cytochrome c activates a protein complex known as
the apoptosome, which triggers the activation of
caspase-9 (Kroemer et al. 2007, Rasola and Bernardi
2007).
Melatonin influences programmed cell death: in
normal cells it exerts an anti-apoptotic effect, in
various cancer cell lines it is pro-apoptotic (Jou et al.
2010). For example, melatonin promotes cell death in
HL-60 cells (Rubio et al. 2007), lymphoma (Trubiani
et al. 2005), HT-29 cell line (Garcia-Navarro et al.
2007) and MCF-7 breast cancer cells (Cucina et al.
2009). The mechanisms by which melatonin acts have
not been completely elucidated, although different
modes of action have been proposed. Melatonin is a
highly lipophilic molecule that readily crosses cell
membranes to reach intracellular organelles,
including mitochondria (Paradies et al. 2010). Indeed,
the evidence points to a melatonin-mitochondria
relationship, and the hormone's anti-apoptotic
properties in healthy cells has been attributed to its
interaction with the mitochondrial transition pore
(Petrosillo et al. 2009, Hibaoui et al. 2009).
Caspase-3 and -9 are the principal mediators of
programmed cell death, and their activation is widely
regarded as an apoptotic marker. Bejarano et al.
(2009) have recently shown that stimulation of HL-60
leukaemia cells with millimolar concentrations of
melatonin increases the activity of both these
caspases, whereas at micromolar concentrations it is
ineffective. These results are coherent with previous
studies that have observed an activation of caspase-3
by melatonin in that same cell line (Rubio et al. 2007)
with the activation occurring via both the extrinsic
and the intrinsic pathways.
Given these antecedents, the aim of the present
work was to study the effect of oral administration of
melatonin on programmed cell death in lymphocytes
and neutrophils, and on various tissues obtained from
rats injected with HL-60 cells.
MATERIAL AND METHODS
Experimental animals
The experimental animals were male Wistar rats
(Rattus norvegicus), 4 to 6 weeks of age, supplied by
the Animalarium Service, University of Extremadura,
housed at a constant temperature of 20±5 °C, and
maintained on "Panlab" feed with water ad libitum.
Four groups were formed: (i) control rats, (ii) control
rats treated with melatonin, (iii) leukaemia cell
injected rats, and (iiii) leukaemia cell injected rats
treated with melatonin. The animals were housed
individually in 25×15×15 inch cages (Panlab), in a
room of 2.86×3.80×2.85 metres, indirectly ventilated,
with 50% relative humidity and artificial lighting, and
were exposed to a 12-hour light/12-hour dark
photoperiod (dark period from 20:00 to 08:00).
HL-60 cells
Rats were injected with a line of human
promyelocytic leukaemia cells (HL-60), cultured in
75 cm3 flasks with RPMI-1640 medium supplemented
with 10% (v/v) foetal bovine serum (heat inactivated),
1.25% DMSO, 1% L-glutamine, 100 U/ml penicillin,
and 100 U/ml streptomycin, at 37 °C and 100%
humidity, in an atmosphere containing 5% CO2. The
cultures presented doubling times of about 48 hours.
Once about 75-80% of confluence had been reached,
the medium was changed under sterile conditions in
a laminar flow hood. Cell counts were performed in
Neubauer chambers, and viability was measured
using trypan blue stain. This stain allows easy
identification of dead cells which take up the dye and
appear blue with uneven cell membranes, while live
cells repel the dye and appear translucent and
colourless.
The treatment solution
A stock solution of melatonin was prepared freshly
every 3 or 4 days containing 348 mg of melatonin
dissolved in 10 ml of 96% ethanol, and stored at
-20 °C. The working solution was prepared by adding
288 microl of this stock solution to 500 ml of water,
giving a final concentration of the hormone of
20 microg/ml. Melatonin (Sigma, St Louis, USA) was
administered in tap water for a period of 16 weeks.
Water bottles were covered with aluminium foil to
protect from light.
Inoculation with HL-60 cells
The rats were inoculated with HL-60 cells by
intraperitoneal injection of 10×107 cells suspended in
2 ml of PBS. A 2-ml syringe was used, with a 0.8×40
mm needle. To obtain the cells, the culture medium
was withdrawn from the flasks, and the cell
suspension was centrifuged in 50 ml Falcon tubes for
5 minutes at 150 g. The supernatant containing the
culture medium was discarded, and the pellet was
resuspended in PBS, adjusting the cell concentration
to that of the study.
Determination of the leukocyte formula
The leukocyte count is based on counting the number
of white blood cells per unit volume present in a
blood sample. The leukocyte formula gives an idea of
the relative proportion of the different types of
leukocytes in a blood sample: neutrophils,
eosinophils, basophils, lymphocytes, and monocytes.
To count the leukocytes, a drop obtained from the
tail was deposited on one end of a slide, and the end
of another slide was placed on this drop until, by
capillary action, it had spread along the edge. A
smear was formed by dragging the top slide over the
other, and the smear was then air-dried.
These samples were fixed in methanol for
5 minutes, and then stained with haematoxylin-eosin
using 5 passes in each of the stains.
Cell counts were made from the stained samples
under 100× oil immersion optical microscopy.
Collection of lymphocytes and polymorphonuclear
cells
The animals of the three groups were anaesthetized
with diethyl ether and killed by decapitation,
collecting the blood immediately from the neck blood
vessels into Falcon tubes containing 0.5 ml of PBS
and 0.5 ml of lithium heparin. Aliquots of 2 millilitres
of this heparinized blood were put into tubes prepared
with two density gradients (separating media) -
Histopaque 1.077 and 1.119. A volume of 2 ml of
1.119 was put into the bottom of each tube, and then
2 ml of 1.077 on top of this, adding it very gently to
avoid mixing. Then the blood was added, again very
gently down the walls of the tube to avoid it mixing
with the density gradients. The preparation was
centrifuged at 600 g for 30 minutes. The lymphocyte
and polymorphonuclear cell rings were collected, and
rinsed twice with PBS (480 g for 10 minutes). The
supernatant was discarded, and the tube with the
precipitate was tapped to separate the cells from the
walls and the bottom of the tube. Finally, the
precipitate was resuspended in 1 ml of Hank's
medium.
Determination of caspase-3 and -9
Once isolated, the lymphocytes and polymorphonuclear cells were subjected to a programmed cell
death treatment with thapsigargin (TG) for 1 hour.
Caspase activity was measured using a peptide
with an aspartic acid residue associated with a
fluorescent compound of the type AMC
(7-amino-4-methylcoumarin). These compounds are
normally of the coumarin type.
After the programmed cell death treatment, the
cell suspension was centrifuged at 500 g for
10 minutes, the supernatant was discarded, and the
precipitate resuspended in 500 microl of lysis buffer. This
suspension was subjected to sonication, with two
4-second pulses at 40%. The application of ultrasound
to the cells ruptures them, releasing the cytosolic
content into the medium. The cells were then
incubated on ice at 4 °C for 15 to 20 minutes, the use
of low temperatures being to halt enzyme activity.
This was followed by centrifugation at 14 000 g for
15 minutes at 4 °C.
To 2 ml of reaction buffer (which contained the
caspase-3 substrate AC-DEVD-AMC or the
caspase-9 substrate AC-LEHD-AMC), 50 microl of the
supernatant were added for the determination of
caspase-3, or 150 microl of supernatant in the case of
caspase-9. The tubes were incubated for 25 to 30
minutes at 37 °C in darkness. During this time,
caspase activity present in the supernatant releases the
AMC fluorophore from the caspase substrate, which
can easily be determined by spectrofluorimetry. The
spectrofluorimeter used was a Shimadzu RF-5301 PC
(Shimadzu Scientific Instruments, Japan), which
accepts samples of 2 ml. In both cases (caspase-3 and
caspase-9), excitation was at 360 nm, and
measurement at 460 nm. Additionally, a blank tube
was measured, containing 5 microl of trial buffer added to
2 ml of reaction buffer. All treatments were
performed in duplicate.
Histological determinations
The dissected organs were fixed in 10% formalin for
one week at 4 °C. They were then rinsed in phosphate
buffered saline (PBS1×) at 4 °C for 2 hours with
mechanical shaking, and dehydrated in increasing
concentrations of ethanol. After treatment in xylene to
make them transparent, they were embedded in
paraffin wax for transversal sectioning. Sections were
cut on a microtome at 7-10 microm, mounted serially on
slides, and kept at 37 °C for one week. Four series of
sections were prepared and distributed on four slides
to ensure the representativeness of the sample.


Fig. 1A, B. Thapsigargin induced caspase activity in neutrophils from control rats, control rats treated with melatonin,
leukaemia cell (HL-60) injected rats, and from leukaemia cell (HL-60) injected rats treated with melatonin. The cells were
incubated with 1 microm thapsigargin (TG) for 60 minutes. The activities of caspase-3 (A) and caspase-9 (B) were estimated as
described in Materials and methods. The data represent the mean ± standard error of 10 separate experiments; (a) statistically
significant as compared with HL-60 injected rats; (b) statistically significant as compared with control animals.
The sections were subjected to a series of changes
in different solutions and for different times
depending on the sample type; in particular, two
changes in xylene, and successive passes in alcohols
of increasing concentration, and distilled water
(dH2O). Two series of sections (two slides) from each
sample were stained with haematoxylin-eosin and two
with Masson trichromate, except the brain samples
for which Nissl staining was used.
Statistical analysis
Data are expressed as mean ± S.E.M. of the number
of determinations carried out in duplicate. The results
were analysed using a non-parametric one-way
ANOVA, followed by a post hoc Tukey test to
compare all pairs of columns, at the significance level
2= alpha0.05.
RESULTS
Figs 1 and 2 show the activity of caspase-3 and
caspase-9 induced by thapsigargin in neutrophils
(Fig. 1) and lymphocytes (Fig. 2).
In neutrophils, the incubation of 1 m thapsi-gargin modified neither caspase-3 (Fig. 1A) nor
caspase-9 (Fig. 1B) activity significantly in the cells
from HL-60 injected rats compared with the control
group. However, these two activities were
significantly reduced in the neutrophils obtained from
the HL-60 injected rats treated with melatonin
compared with those not treated with the hormone.
Similarly, the incubation of 1 m thapsigargin
modified neither caspase-3 (Fig. 2A) nor caspase-9
(Fig. 2B) activity significantly in the lymphocytes
from HL-60 injected rats compared with the control
group. Again however, treatment with melatonin significantly reduced these two activities in the
lymphocytes in response to the thapsigargin,
compared with the HL-60 injected rats not treated
with the hormone.


Fig. 2A, B. Thapsigargin induced caspase activity in lymphocytes from control rats, control rats treated with melatonin,
leukaemia cell (HL-60) injected rats, and from leukaemia cell (HL-60) injected rats treated with melatonin. The cells were
incubated with 1 microm thapsigargin (TG) for 60 minutes. The activities of caspase-3 (A) and caspase-9 (B) were estimated as
described in Materials and methods. The data represent the mean ± standard error of 10 separate experiments; (a) statistically
significant as compared with HL-60 injected rats; (b) statistically significant as compared with controls.
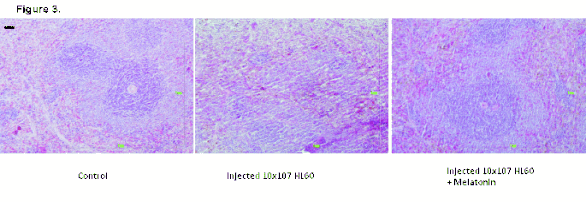
Fig. 3. Detail of a follicle of the spleen of a control rat, a leukaemia cell (HL-60) injected rat, and a leukaemia cell injected rat
treated with melatonin. The stain used is haematoxylin-eosin. Scale Bar = 120 microm.
Table 1. Percentage values of the leukocyte count obtained after staining a drop of blood from control rats, control rats
treated with melatonin, leukaemia cell (HL-60) injected rats, and leukaemia cell injected rats treated with melatonin.
|
CONTROL |
MEL |
HL-60 |
HL-60 + MEL | | LYMPHOCYTES |
0.64±0.02 |
0.61±0.02 |
0.79±0.01a, b |
0.60±0.01 | | NEUTROPHILS |
0.34±0.02 |
0.36±0.02 |
0.15±0.01a, b |
0.39±0.02 | | EOSINOPHILS |
0.02±0.01 |
0.01±0.01 |
0.06±0.02a, b |
0.01±0.01 |
Each value represents the mean ± standard error of 10 determinations performed in the last week of the study prior to killing the
animals; (a) statistically significant versus the control group; (b) statistically significant versus animals treated with both
HL-60 and melatonin.
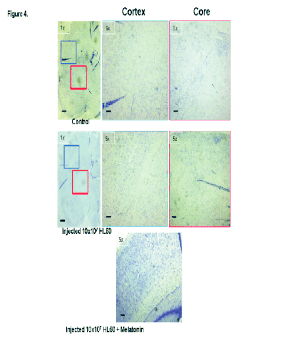
Fig. 4. Cortex and core of brain samples from a control rat, a leukaemia cell (HL-60) injected rat, and a leukaemia cell injected
rat treated with melatonin. The stain used is cresyl violet (Nissl stain). Scale Bar = 500 microm (5×), 2 mm (1×).
Table 1 lists the percentages of the leukocyte
formulas of blood obtained from control rats, control
rats treated with melatonin, leukaemia cell (HL-60)
injected rats, and leukaemia cell (HL-60) injected rats
treated with melatonin. The lymphocyte and
eosinophil percentages in the group of leukaemia cell
(HL-60) injected rats were both significantly greater
than in the control group and in the leukaemia cell
(HL-60) injected rats treated with melatonin. In
contrast, there was a significant decrease (statistically
significant) in the neutrophil percentage in the blood
obtained from the leukaemia cell (HL-60) injected rats
with respect to the control group and the group of
leukaemia cell (HL-60) injected rats treated with the
hormone.
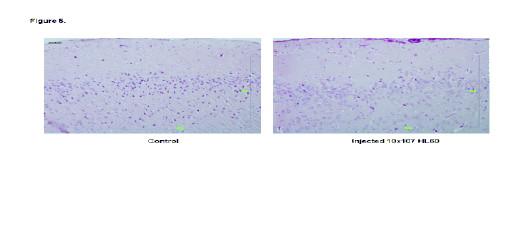
Fig. 5. Brain samples from a control rat, a leukaemia cell (HL-60) injected rat, and a leukaemia cell injected rat treated with
melatonin. The stain used is haematoxylin-eosin. Scale Bar = 120 microm.
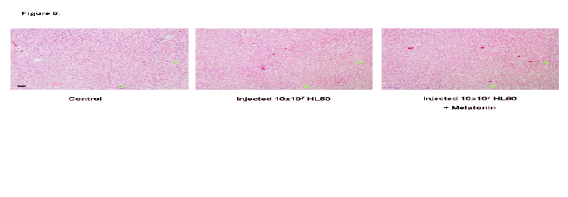
Fig. 6. Liver samples from a control rat, a leukaemia cell (HL-60) injected rat, and a leukaemia cell injected rat treated with
melatonin. The stain used is haematoxylin-eosin. Scale Bar = 120 microm.

Fig. 7. Histological sections of testes of a control rat, a leukaemia cell (HL-60) injected rat, and a leukaemia cell injected rat
treated with melatonin. The stain used is haematoxylin-eosin. Scale Bar = 120 microm.
Fig. 3 corresponds to the spleen. The staining reveals
the white pulp (lymphoid tissue with a bluish colour
because the nucleus occupies 90% of the cytoplasm)
and the red pulp (mainly erythrocytes, enucleated
cells of a more reddish colour). There are no striking
changes in this distribution between the three groups,
but one observes in the group of animals injected with
HL-60 a decrease in size (atrophy) of the lymphoid
follicles relative to the control, but not in the case of
the animals treated with melatonin. This could
correspond to a side effect of the treatment, such as
weight loss.
The focus of the study of the brain specimens was
on the regions of the subarachnoid space and the
spaces of Virchow as being where the appearance of
infiltration starts and is most often observed (Figs 4,
5). No involvement was observed of tissue-level
structures such as basal ganglia or cortex.
Nor in the liver samples (Fig. 6) were any striking
histological differences observed between the three
groups. There was no appreciable increase in collagen
(green) which would have been indicative of tissue
damage, and the typical histological structures were
conserved.
The histological analysis of the testes (Fig. 7)
showed no significant differences between the three
groups, the disposition of the seminiferous tubules
being normal, and the cellularity conserved.
DISCUSSION
Melatonin is a molecule that has been well conserved
evolutionarily (Tan et al. 2010), is ubiquitous and
plays important roles in various physiological
processes. Among the physiological effects of this
indolamine are the control of circadian and
activity-rest and sleep-wake rhythms, and of seasonal
reproductive cycles. It also has antioxidant and
immunomodulatory activity (Rodriguez et al. 2005,
Chahbouni et al. 2010). In cancer treatment, this
indolamine is involved in controlling tumour
development and growth, presenting an anti-
proliferative activity in numerous cancer cell lines
(Garcia-Navarro et al. 2007, Wenzel et al. 2005).
There have been only a few studies with leukaemia
cells, however. In particular, since the
anti-proliferative and pre-apoptotic effects of
melatonin have been described for leukaemic cells
only using in vitro models (Buyukavci et al. 2006,
Rubio et al. 2007, Bejarano et al. 2009), the present
investigation was designed to determine the effect of
melatonin on the caspase activity of lymphocytes and
neutrophils obtained from Wistar rats injected with
the cell line HL-60.
HL-60 cells constitute a leukaemia cell line that
has been used for research on how certain types of
blood cells are formed. HL-60 cells are also widely
investigated to determine the effect of DNA
topoisomerase (topo) IIalpha and IIbeta on cell
differentiation and programmed cell death (Sugimoto
et al. 1998) and is especially useful in studies of
dielectrophoresis (Ratanachoo et al. 2002) which
require an aqueous environment with round cells in
suspension. These cells have also made contributions
in studies of programmed cell death and intracellular
Ca2+ homeostasis (Fang et al. 1998).
Recent reports have documented that treatment
with melatonin inhibits programmed cell death in
non-tumour cells (Jou et al. 2010), while others have
suggested that this indolamine promotes programmed
cell death in cancer cells (Sainz et al. 2003). In this
sense, our results have shown that, in neutrophils and
lymphocytes obtained from leukaemia cell injected
rats treated with melatonin, the TG-induced caspase-3
and caspase-9 activities are lower than those in
neutrophils and lymphocytes obtained from injected
rats untreated with the indolamine. We also observed
a reduction of caspase-9 activity in neutrophils and of
caspase-3 activity in lymphocytes obtained from
leukaemia cell injected rats treated with melatonin
relative to the controls. This reduction in caspase
activity could be due to the known anti-apoptotic
processes promoted by melatonin in immune cells
(Sainz et al. 2003).
In this regard, there has recently been reported a
decrease in TG- and fMLP- (N-formyl-methionyl-leucyl-phenyalanine) induced programmed cell death
in human neutrophils and lymphocytes treated with
melatonin (Espino et al. 2010) as has also been
described by other workers (Luchetti et al. 2006,
Radogna et al. 2008). It appears that melatonin exerts
this protective action by blocking the opening of the
mPTP (Espino et al. 2010). This same anti-apoptotic
process has been described for other cell types:
kidney cells (Kunduzova et al. 2003), hippocampal
neurons (Shen et al. 2002), cultured mouse striatal
neurons (Andrabi et al. 2004), pineal cells (Yoo et al.
2002) and cardiomyocytes (Petrosillo et al. 2009).
The inductor of programmed cell death, TG, is a
specific inhibitor of the Ca2+-ATPase of the
endoplasmic reticulum, SERCA, with which it blocks
the refilling of intracellular Ca2+ stores, inducing an
increase in the cytosolic Ca2+ concentration, and thus
causing an overload of Ca2+ in the mitochondria.
When the mitochondria are overloaded with Ca2+,
mitochondrial uncoupling occurs, accompanied by a
depolarization of the inner mitochondrial membrane
and the production of ROSs (Reactive Oxygen
Species) of mitochondrial origin. Also, the
mitochondrial permeability transition pore opens,
constituting a megachannel that allows large
molecular weight molecules to pass (Korsmeyer et al.
2000). The opening of this pore facilitates the release
of pro-apoptotic agents of mitochondrial origin, such
as cytochrome c and the Apaf-1 protein, among
others, which together with procaspase-9 form a
multimolecule complex known as the "apoptosome".
This activates caspase-9, an initiator caspase, which
in turn activates other executor caspases including
caspase-3, triggering the process of programmed cell
death (Korsmeyer et al. 2000) and resulting in cell
death. In the current study, in the cells obtained from
rats treated with melatonin, this process was
inhibited, since melatonin blocks the opening of the
mPTP, which would explain the decrease of
caspase-3 and -9 activity we observed in lymphocytes
and neutrophils (Kroemer et al. 2007, Rasola and
Bernardi 2007).
We also recorded changes in leukocyte counts (the
leukocyte formula), with the percentage of
lymphocytes in the group of leukaemia cell (HL-60)
injected rats treated with melatonin being lower than
both the control group and the untreated group of
injected rats, while the percentage of neutrophils was
higher than in the other two groups. There was also
an increase of eosinophils in the blood of the
leukaemia cell (HL-60) injected rats with respect to
the other two groups. These facts may reflect the
existence of a population of HL-60 cells in the
injected animals that, while insufficient to trigger a
typical pathology of leukaemia, is capable of causing
the atrophy of some tissues, as was observed in the
histological study of the spleen.
In HL-60 injected rats, melatonin functions as an
inducer of programmed cell death and an inhibitor of
tumour development. This was observed in Ehrlich
ascites carcinoma (EAC) cells which were injected
intraperitoneally into female mice (El-Missiry and
Abd El-Aziz 2000), in which oral administration of
melatonin reduced the viability and volume of the
tumour, and delayed the progression of the cell cycle.
Also in rats with colon cancer, melatonin reduced
both the multiplicity of tumours and the mitotic index
(Anisimov et al. 2000).
Previous in vitro studies have shown that
melatonin has a time-dependent effect in promoting
the programmed cell death of HL-60 cells (Bejarano
et al. 2009), confirming earlier results of Rubio et al
(2007). Similar findings have been reported for other
cell types: B-lymphoma cells (Trubiani et al. 2005,
Garcia-Navarro et al. 2007), human HT-29 colorectal
cancer cells (Wenzel et al. 2005), HepG2 hepatocarcinoma cells (Martin-Renedo et al. 2008), the
colon 38 cancer cell line (Melen-Mucha et al. 1998)
and rat pituitary prolactin-secreting tumour cells
(Yang et al. 2007).
Rubio et al. (2007) also observed the effect of
melatonin on cell viability, and found that high
concentrations of melatonin (in the millimolar range)
significantly diminished the reduction of MTT
(3-[4,5/dimethyl/thiazol/2/yl]-2,5 diphenyl trihydro-chloride) staining and the number of HL-60 cells.
Bejarano et al. (2009) report a time-dependent
activation-deactivation of the caspase-9 initiator
induced by melatonin treatment, with the maximum
effect being 12 hours after stimulation.
Overall, our results show that melatonin plays a
crucial role in the inhibition of lymphocyte and
neutrophil caspase-3 and -9 activity in control rats, as
well as providing the capacity to revert to the basal
lymphocyte, neutrophil, and eosinophil proportions in
HL-60 injected rats.
ACKNOWLEDGEMENTS
This research was supported by MEC-DGI and Junta
de Extremadura grants BFU2007-60091 and
PRI07-A024, respectively. The authors would like to
express their thanks to Ms Elena Circujano Vadillo
for her technical assistance. The two first authors
contributed equally to this work.
REFERENCES
Andrabi SA, Sayeed I, Siemen D, Wolf G, Horn TF. Direct inhibition of the mitochondrial permeability transition pore: a possible mechanism responsible for anti-apoptotic effects of melatonin. FASEB J. 18: 869-871, 2004.
[CrossRef]
Anisimov VN, Popovich IG, Shtylik AV, Zabezhinski MA, Ben-Huh H, Gurevich P, Berman V, Tendler Y, Zusman I. Melatonin and colon carcinogenesis III. Effect of melatonin on proliferative activity and apoptosis in colon mucosa and tumours induced by 1,2-dimethylhydrazine in rats. Exp Toxicol Pathol. 52: 71-76, 2000.
Bejarano I, Redondo PC, Espino J, Rosado JA, Paredes SD, Barriga C, Reiter RJ, Pariente JA, Rodriguez AB. Melatonin induces mitochondrial-mediated apoptosis in human myeloid HL-60 cells. J Pineal Res. 46: 392-400, 2009.
[CrossRef]
Berger J. A two-clock model of circadian timing in the immune system of mammals. Pathol Biol. 56: 286-291, 2008.
[CrossRef]
Buyukavci M, Ozdemir O, Buck S, Stout M, Ravindranth Y, Savasan S. Melatonin cytotoxicity in human leukemia cell: relation with its pro-oxidant effect. Fundam Clin Pharmacol. 20: 73-79, 2006.
[CrossRef]
Carrillo-Vico A, Lardone PJ, Fernandez-Santos JM, Martin-Lacave I, Calvo JR, Karasek M, Guerrero JM. Human lymphocyte-synthesized melatonin is involved in the regulation of the interleukin-2/interleukin-2 receptor system. J Clin Endocrinol Metab. 9: 992-1000, 2005.
[CrossRef]
Chahbouni M, Escames G, Venegas C, Sevilla B, Garcia JA, Lopez LC, Munoz-Hoyos A, Molina-Carballo A, Acuna-Castroviejo D. Melatonin treatment normalizes plasma pro-inflammatory cytokines and nitrosative/oxidative stress in patients suffering from duchenne muscular dystrophy. J Pineal Res. 48: 282-289, 2010.
[CrossRef]
Cos S, Garcia-Bolado A, Sanchez-Barcelo EJ. Direct antiproliferative effects of melatonin on two metastatic cell sublines of mouse melanoma (B16BL6 and PG19). Melanoma Res. 11: 197-201, 2001.
Cos S, Gonzalez A, Guezmes A, Mediavilla MD, Martinez-Campa C, Alonso-Gonzalez C, Sanchez-Barcelo EJ. Melatonin inhibits the growth of DMBA-induced mammary tumours by decreasing the local biosynthesis of estrogens through the modulation of aromatase activity. Int J Cancer. 118: 274-278, 2006.
[CrossRef]
Cucina A, Proietti S, D'Anselmi F, Coluccia P, Dinicola S, Frati L, Bizzarri M. Evidence for a biphasic apoptotic pathway induced by melatonin in MCF-7 breast cancer cells. J Pineal Res. 46: 172-180, 2009.
[CrossRef]
Dauchy RT, Blask DE, Dauchy EM, Davidson LK, Tirrell PC, Greene MW, Tirrell RP, Hill CR, Sauer LA. Antineoplastic effects of melatonin on a rare malignancy of mesenchymal origin: melatonin receptor-mediated inhibition of signal transduction, linoleic acid metabolism and growth in tissue-isolated human leiomyosarcoma xenografts. J Pineal Res. 47: 32-42, 2009.
[CrossRef]
El-Missiry MA, Abd El-Aziz AF. Influence of melatonin on proliferation and antioxidant system in Ehrlich ascites carcinoma cells. Cancer Lett. 151: 119-125, 2000.
Espino J, Bejarano I, Redondo PC, Rosado JA, Barriga C, Reiter JR, Pariente JA, Rodriguez AB. Melatonin reduces apoptosis induced by calcium signaling in human leukocytes: Evidence for the involvement of mitochondria and Bax activation. J Membr Biol. 233: 105-118, 2010.
[CrossRef]
Fang M, Zhang H, Xue S. Role of calcium in apoptosis of HL-60 cells induced by harringtonine. Sci China C Life Sci. 41: 600-607, 1998.
[CrossRef]
Garcia-Navarro A, Gonzalez-Puga C, Escames G, Lopez LC, Lopez A, Lopez-Cantarero M, Camacho E, Espinosa A, Gallo MA, Acuna-Castroviejo D. Cellular mechanisms involved in the melatonin inhibition of HT-29 human colon cancer cell proliferation in culture. J Pineal Res. 43: 195-205, 2007.
[CrossRef]
Hajnoczyk G, Davies E, Madesh M. Calcium signaling and apoptosis. Biochem Biophys Res Commun. 304: 445-454, 2003.
Hardeland R, Fuhrberg B. Ubiquitous melatonin-presence and effects in unicells, plants and animals. Trends Comp Biochem Physiol. 2: 25-45, 1996.
Hibaoui Y, Roulet E, Ruegg UT. Melatonin prevents oxidative stress-mediated mitochondrial permeability transition and death in skeletal muscle cells. J Pineal Res. 47: 238-252, 2009.
[CrossRef]
Jou MJ, Peng TI, Hsu LF, Jou SB, Reiter RJ, Yang CM, Chiao CC, Lin YF, Chen CC. Visualization of melatonin's multiple mitochondrial levels of protection against mitochondrial Ca(2+)-mediated permeability transition and beyond in rat brain astrocytes. J Pineal Res. 48: 20-38, 2010.
[CrossRef]
Kobayashi H, Kromminga A, Dunlop TW, Tychsen B, Conrad F, Suzuki N, Memezawa A, Bettermann A, Aiba S, Carlberg C, Paus R. A role of melatonin in neuroectodermal-mesodermal interactions: the hair follicle synthesizes melatonin and expresses functional melatonin receptors. FASEB J. 19: 1710-1712, 2005.
[CrossRef]
Korsmeyer SJ, Wei MC, Saito M, Weiler S, Oh KJ, Schlesinger PH. Pro-apoptotic cascade activates BiD, which oligomerizes Bak or Bax into pores that result in the release of cytochrome c. Cell Death Differ. 7: 1166-1173, 2000.
[CrossRef]
Kroemer G, Galluzzi L, Brenner C. Mitochondrial membrane permeabilization in cell death. Physiol Rev. 87: 99-163, 2007.
[CrossRef]
Kunduzova OR, Escourrou G, Seguelas MH, Delagrange P, De La Farge F, Cambon C, Parini A. Prevention of apoptotic and necrotic cell death, caspase-3 activation, and renal dysfunction by melatonin after ischemia/reperfusion. FASEB J. 17: 872-874, 2003.
[CrossRef]
Luchetti F, Canonico B, Curci R, Battistelli M, Mannello F, Papa S, Tarzia G, Falcieri E. Melatonin prevents apoptosis induced by UV-B treatment in U937 cell line. J Pineal Res. 40: 158-167, 2006.
[CrossRef]
Martin-Renedo J, Mauriz JL, Jorquera F, Ruiz-Andres O, Gonzalez P, Gonzalez-Gallego J. Melatonin induces cell cycle arrest and apoptosis in hepatocarcinoma HepG2 cell line. J Pineal Res. 45: 532-540, 2008.
[CrossRef]
Melen-Mucha G, Winczyk K, Pawlikowski M. Somatostatin analogue oxtreotide and melatonin inhibit bromodeoxyuridine incorporation into cell nuclei and enhance apoptosis in the transplantable murine colon 38 cancer. Anticancer Res. 18: 3615-3620, 1998.
Panke ES, Rollag MD, Reiter RJ. Pineal melatonin concentrations in the Syrian hamster. Endocrinology. 104: 194-197, 1979.
Paradies G, Petrosillo G, Paradies V, Reiter RJ, Ruggiero FM. Melatonin, cardiolipin and mitochondrial bioenergetics in health and disease. J Pineal Res. 48: 297-310, 2010.
[CrossRef]
Park SY, Jang WJ, Yi EY, Jang JY, Jung Y, Jeong JW, Kim YJ. Melatonin suppresses tumour angiogenesis by inhibiting HIF-1alpha stabilization under hypoxia. J Pineal Res. 48: 178-184, 2010.
Petrosillo G, Moro N, Ruggiero FM, Paradies G. Melatonin inhibits cardiolipin peroxidation in mitochondria and prevents the mitochondrial permeability transition and cytochrome c release. Free Radic Biol Med. 47: 969-974, 2009.
[CrossRef]
Radogna F, Cristofanon S, Paternoster L, D'Alessio M, De Nicola M, Cerella C, Dicato M, Diederich M, Ghibelli L. Melatonin antagonizes the intrinsic pathway of apoptosis via mitochondrial targeting of Bcl-2. J Pineal Res. 44: 316-325, 2008.
[CrossRef]
Rasola A, Bernardi P. The mitochondrial permeability transition pore and its involvement in cell death and in disease pathogenesis. Apoptosis. 12: 815-833, 2007.
[CrossRef]
Ratanachoo K, Gascoyne PRC, Ruchirawat M. Detection of cellular responses to toxicants by dielectrophoresis. BBA. 1564: 449-458, 2002.
Reiter RJ. Pineal control of a seasonal reproductive rhythm in male golden hamsters exposed to natural daylight and temperature. Endocrinology. 92: 423-430, 1973.
Reiter RJ. Pineal melatonin: cell biology of its synthesis and of its physiological interaction. Endocr Rev. 12: 151-180, 1991.
[CrossRef]
Reiter RJ, Fraschini F. Endocrine aspects of the mammalian pineal gland: a review. Neuroendocrinology. 5: 219-255, 1969.
Rodriguez AB, Barriga C, Paredes SD, Terron MP. Age, melatonin and the immune system. In Pandalai SG (ed.): Recent Research Developments in Molecular and Cellular Biochemistry, Vol. 2, Part II., Research SignPost, 2005, pp. 255-287.
Rubio S, Estevez F, Cabrera J, Reiter RJ, Loro J, Quintana J. Inhibition of proliferation and induction of apoptosis by melatonin in human myeloid HL-60 cells. J Pineal Res. 42: 131-138, 2007.
[CrossRef]
Sainz RM, Mayo JC, Rodriguez C, Tan DX, Lopez-Burillo S, Reiter RJ. Melatonin and cell death: differential actions on apoptosis in normal and cancer cells. Cell Mol Life Sci. 60: 1407-1426, 2003.
[CrossRef]
Scaffidi C, Fulda S, Srinivasan A, Friesen C, Li F, Tomaselli KJ, Debatin KM, Krammer PH, Peter ME. Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 17: 1675-1687, 1998.
[CrossRef]
Shen YX, Xu SY, Wei W, Wang XL, Wang H, Sun X. Melatonin blocks rat hippocampal neuronal apoptosis induced by amyloid beta-peptide 25-35. J Pineal Res. 32: 163-167, 2002.
Sugimoto K, Yamada K, Egashira M, Yazaki Y, Hirai H, Kikuchi A, Oshimi K. Temporal and spatial distribution of DNA topoisomerase II alters during proliferation, differentiation, and apoptosis in HL-60 cells. Blood. 91: 1407-1417, 1998.
Tan DX, Hardeland R, Manchester LC, Paredes SD, Korkmaz A, Sainz RM, Mayo JC, Fuentes-Broto L, Reiter RJ. The changing biological roles of melatonin during evolution: from an antioxidant to signals of darkness, sexual selection and fitness. Biol Rev Camb Philos Soc. 85: 607-623, 2010.
[CrossRef]
Trubiani O, Recchioni R, Moroni F, Pizzicannella J, Caputi S, Di Primio R. Melatonin provokes cell death in human B-lymphoma cells by mitochondrial-dependent apoptotic pathways activation. J Pineal Res. 39: 425-431, 2005.
[CrossRef]
Wenzel U, Nickel A, Daniel H. Melatonin potentiates flavone-induced apoptosis in human colon cancer cells by increasing the level of glycolytic end products. Int J Cancer. 116: 236-242, 2005.
[CrossRef]
Yang QH, Xu JN, Xu RK, Pang SF. Antiproliferative effects of melatonin on the growth of rat pituitary prolactin-secreting tumour cells in vitro. J Pineal Res. 42: 172-179, 2007.
[CrossRef]
Yoo YM, Yim SV, Kim SS, Jang HY, Lea HZ, Hwang GC, Kim JW, Kim SA, Lee HJ, Kim CJ, Chung JH, Leem KH. Melatonin suppresses NO-induced apoptosis via induction of Bcl-2 expression in PGT-beta immortalized pineal cells. J Pineal Res. 33: 146-150, 2002.
|
BACK
|










