Journal of APPLIED BIOMEDICINE
ISSN 1214-0287 (on-line)
ISSN 1214-021X (printed)
Volume 9 (2011), No 4, p 209-218
DOI 10.2478/v10136-011-0007-2
Phosphorylation of histone H2AX in peripheral blood mononuclear cells after thoracic irradiation of rats
Radim Havelek, Martina Rezacova, Zuzana Sinkorova, Lenka Zarybnicka, Jaroslav Pejchal, Jirina Vavrova
Address: Radim Havelek, Department of Medical Biochemistry, Faculty of Medicine in Hradec Kralove, Simkova 870, 500 38 Hradec Kralove, Czech Republic
havelekr@lfhk.cuni.cz
Received 23rd February 2011.
Revised 28th April 2011.
Published online 30th June 2011.
Full text article (pdf)
Summary
Key words
Introduction
Material and Methods
Results
Discussion
Acknowledgement
References
SUMMARY
Lymphocytes are among the most radiosensitive cells. After exposure of the organism to ionizing radiation, they promptly die by apoptosis at a rate proportional to the dose received. Because of this, they are frequently used in biodosimetry. We demonstrated that one hour after whole-body irradiation of rats, histone H2AX in the lymphocyte nuclei was quickly phosphorylated on serine 139, the phosphorylation process being directly dependent on the gamma radiation dose. In the work presented here, we studied the kinetics of lymphocyte depletion in the peripheral blood and phosphorylation of histone H2AX in the peripheral blood lymphocytes after local (thoracic) irradiation of rats. Twenty-four hours after whole-body irradiation of the rats at a dose of 5 Gy, the lymphocyte count declined to almost zero values, whereas after local irradiation of the thorax area, the counts of lymphocytes in the peripheral blood remained unaltered.
The authors employed two methods (flow-cytometric and microscopic) for the gammaH2AX determination in the peripheral blood lymphocytes, 1 h after thoracic irradiation of rats. Flow cytometry revealed a dose dependence on the increase in gammaH2AX in a dose range of 10-30 Gy. The microscopic method was more sensitive in the case of lower radiation doses, the dependence on the dose being obvious from a dose as low as 5 Gy. The methods are able, in the dose range 5-30 Gy, to differentiate between the type of irradiation, i.e. the whole-body or local.
KEY WORDS
gammaH2AX; biodosimetry; ionizing radiation; thoracic; lymphocytes
INTRODUCTION
In radiation accidents, it is frequently impossible to
establish doses in the people exposed based on
physical dosimetry and thus, there is a need for new
biodosimetric indicators which can be used in the
retrospective analysis of the doses received. At the
present time, the method frequently used is to
determine the dicentric chromosomes in lymphocytes
isolated from the peripheral blood of the radiation
exposed persons (Blakely et al. 2009). The method,
however, has certain limitations, particularly the long
time lapse between the exposure and dose
determination due to the necessity to incubate the
lymphocytes with phytohaemagglutinin.
Exposure of lymphocytes to increasing doses
(under in vivo as well as in vitro conditions) results in
their exponential cellular death by apoptosis. Under
in vivo conditions, apoptotic cells are quickly
phagocytosed and destroyed by the reticulo-endothelial system. The maximum decrease in the
number of lymphocytes in the peripheral blood of
whole-body irradiated animals has been observed
72 hours after irradiation (Vavrova and Filip 2002),
and the predictability of the rate of lymphocyte
depletion has made it possible to develop a model for
purposes of biodosimetry based on lymphocyte
depletion kinetics (Goans et al.1997).
Ionizing radiation (IR) produces DNA
double-strand breaks (DSB) and, at the site of the
DSB, so called ionizing radiation-induced foci (IRIF)
are formed. After DSB generation, the neighbouring
chromatin undergoes extensive modifications,
initiated by the ataxia telangiectasia mutated (ATM) -
mediated phosphorylation of histone H2AX (gamma-H2AX) (Rogakou et al. 1998) followed by the
recruitment of MDC1 adaptor and two ubiquitin
ligases, RNF8 and RNF168 (Larsen et al. 2010).
Thereafter, MRN complex repair proteins (NBS1,
MRE11 a Rad50), BRCA1 and 53BP occur at the
DSB (Bekker-Jensen et al. 2006). H2AX is a member
of the H2A family and accounts for from 2% to 25%
of the total histone H2A pool. Histone H2AX
contains a distinct C-terminal extension with serine as
the 4th C-terminal amino acid. This serine is
phosphorylated after the DSB induction and the
phosphorylated histone H2AX has been named
gamma H2AX. In human H2AX, this C-terminal
serine is serine 139 (Rogakou et al. 1998). Gamma
H2AX could be specifically detected as early as a few
minutes after the DSB induction (Huang and
Darzynkiewicz 2006).
In our previous studies (Vilasova et al. 2008), we
demonstrated the dose-dependent phosphorylation of
H2AX 1 hour after in vitro irradiation of a human
acute lymphoblastic leukemia cell line (MOLT-4) and
human peripheral blood lymphocytes at doses of 0.5
to 5 Gy. We also demonstrated a dose dependence of
the H2AX phosphorylation in lymphocytes isolated
from the peripheral blood of whole-body irradiated
rats one hour after irradiation. Specimens of
peripheral blood may be stored for 23 hours on ice in
a refrigerator (4 °C) to make possible a delayed
sample analysis with a minimum level of gammaH2AX
decay (Havelek et al. 2011).
However, partial-body irradiation (from external
sources) of humans is more common than whole-body
irradiation. The lungs are organ sensitive to gamma
radiation exposure. Lungs exposed to a gamma
radiation dose over 8 Gy suffer from acute radiation
pneumonitis as well as chronic radiation fibrosis in
later periods of time after irradiation. Radiation
pneumonitis is an exudative inflammation, which
occurs in irradiated areas only (Travis 1980).
Differences were described between the responses of
different strains of mice and rats to thoracic
irradiation (Down 1986, Jackson et al. 2010). Jackson
et al. (2010) compared the responses of C57L and
C57BL6 mice to thoracic irradiation at doses of
10-15 Gy. The C57L mice were very sensitive to the
development of early pneumonitis 3-4 months after
irradiation; in C57Bl6 mice, the response was delayed
and many mice accumulated large amounts of the
pleural fluid in the lungs 6-9 months after irradiation;
Wistar rats developed a combination of early pleural
effusions and pneumonitis. Osterreicher et al. (2004)
studied the dose-dependent response in rats three
weeks after irradiation at doses of 1-25 Gy. A
significant dose-dependent depletion of type II
pneumocytes was found after thoracic irradiation of
the rats at a dose of 1 Gy and above. Alveolar
neutrophils increased in number after 1 Gy with a
dose dependence observed after 10-25 Gy and
alveolar septa thickening after 5-25 Gy.
In the work presented here we studied the
possibility of using the gammaH2AX measurement as a
biodosimetric indicator after partial-body irradiation.
Gamma radiation exposure of the thoracic region was
used as a model example of partial-body irradiation.
Changes in gammaH2AX expression in the lymphocytes of
rats after in vivo local thoracic irradiation at doses of
5-30 Gy, observed by flow cytometry and
immunocytochemistry, were analysed to
retrospectively estimate the gamma radiation doses
received.
MATERIAL AND METHODS
Animals
SPF (specific pathogen-free) female Wistar rats
(VELAZ-Lysolaje, Czech Rep.), body weight of
210-250 g, were used. Each experimental group
included 6 animals. The animals were housed in a
temperature- and humidity-controlled environment
with a 12-hour light/dark cycle. Food and water were
available ad libitum. All the procedures were
approved by the Ethical Committee supervising
experiments performed on animals at the Faculty of
Military Health Sciences Hradec Kralove (MO
12-7/2008-3696).
Gamma irradiation
The female Wistar rats were exposed to whole-body
(5 Gy) and local thoracic (5, 10, 20 and 30 Gy)
irradiation by using a 60Co gamma-ray source (Chisotron
Chirana, Czech Rep.) at a distance from the source of
0.5 m and dose rate of 3 Gy/min. The doses were
measured by an ionization chamber (Dosemeter PTW
Unidos 1001, serial No. 11057, with ionization
chamber PTW TM 313, serial No. 0012; RPD Inc.,
USA); the set was validated by the Czech Metrology
Institute - Inspectorate for Ionizing Radiation
(protocol No. 9011-OL-U4124/2005). The animals
were slightly anaesthetized before irradiation with a
mixture of Rometar (10 mg/kg) and Narkamon
(1.2 mg/kg) (Bioveta, Czech Rep.). The solution was
injected intramuscularly. Local thoracic irradiation
was performed in a field 3 cm wide; the body was
otherwise shielded by a 10-cm lead layer, which
reduced the dose in the remaining parts of the body to
about 2-3% of the lung dose (Osterreicher et al.
2004). Control animals were treated in the same way,
but were not irradiated.
Leukocyte differential count
A peripheral blood sample (5-7 ml) was taken from
the rat myocardium (one sample from each animal)
and the differential count was determined on the
Sysmex XE-2100 haematological analyzer
(Sysmex-Toa, Japan).
Flow-cytometric detection of gammaH2AX
The method of Huang and Darzynkiewicz (2006) was
modified and used for gammaH2AX detection. The method
was optimized for the detection of gammaH2AX in the rat
peripheral blood mononuclear cells (PBMCs). The
PBMCs of the rats exposed to thoracic irradiation and
controls were isolated with Histopaque (Sigma
Aldrich, Germany) according to the manufacturer's
instructions. The red blood cells trapped in pellets
were lysed with the EasyLyseTM reagent
(DakoCytomation, Germany) according to the
manufacturer's instructions. The PBMCs were rinsed
twice with PBS (Dulbecco's phosphate buffered
saline, Sigma-Aldrich, Germany) and then fixed with
an ice-cold 1% methanol-free formaldehyde solution,
rinsed with PBS and suspended in ice-cold 70%
ethanol. The cells were stored at 4 °C before further
manipulation. In the next step, the cells were rinsed
with 1% BSA-0.2%-Triton X-100 in PBS and stained
with anti-phospho histone H2AX (Ser139)-FITC
conjugate primary antibody (Millipore, USA) at room
temperature for 1 h in the dark. The cells (after
rinsing) were suspended in a propidium iodide (PI)
staining solution (0.1% RNase, PI 5microg/mL in PBS),
incubated at room temperature for 30 min in the dark
with PI staining solution and analysed immediately
after incubation, on the FACS analyser CyAn
DakoCytomation (Beckman Coulter, USA). At least
100 000 cells were analysed per each sample. List
mode data were analysed using the Summit v 4.3
software (Beckman Coulter, USA). The cells in
G0/G1 phase were gated and the mean of
fluorescence of the cells in each sample was
calculated. Blood samples from 6 animals were
analysed per experimental dose.
Immunocytochemical detection of gammaH2AX
The PBMCs were isolated as described above and
fixed with 4% freshly prepared paraformaldehyde for
10 min at room temperature, washed with PBS,
permeabilized in 0.2% Triton X-100/PBS for 15 min
at room temperature, and washed with PBS. Before
the incubation with primary antibodies (overnight at
4 °C), the cells were incubated with 7% inactivated
foetal calf serum + 2% bovine serum albumin in PBS
for 30 min at room temperature. Murine monoclonal
anti-phospho-histone H2AX (Millipore, USA) was
used for gammaH2AX detection. For the secondary
antibody, the affinity pure donkey anti-mouse-FITC-conjugated antibody was purchased from the
Jackson Laboratory (USA). The secondary antibody
was applied to each slide (after their pre-incubation
with 5.5% donkey serum in PBS for 30 min at room
temperature), incubated for 1 h in dark and washed
(3×5 min) with PBS. The nuclei were counterstained
with 4',6-diamidino-2-phenylindole (DAPI). Images
were obtained by the Nikon Eclipse fluorescence
microscope; the exposure time and dynamic range of
the camera in all the channels were adjusted to the
same values for all the slides to obtain quantitatively
comparable images.
Statistics
The bivariate histograms of gammaH2AX-FITC
immunofluorescence intensity versus DNA content
distributions obtained by the flow cytometer were
analysed by using the Summit v 4.3 software
(Beckman Coulter, USA) and the mean of
immunofluorescence intensities of gammaH2AX-FITC
signals in nuclei of lymphocytes in each sample was
calculated by using this software. The descriptive
statistics of the results was calculated and the charts
were made in Microsoft Office Excel 2003 (Microsoft
Inc., USA) or GraphPad Prism 5 biostatistics
(GraphPad Software, Inc., USA). In this study, all the values were expressed as arithmetic means with
S.E.M of hexaplicates unless otherwise noted. The
significant differences between the groups were
analysed by the Student's t-test at the significance
level 2alpha=0.05.
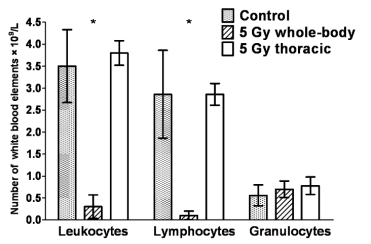
Fig. 1. Comparison of leukocyte differential counts in rats 24 h following in vivo whole-body and thoracic irradiation at
a dose of 5 Gy. The figure presents arithmetic mean values (× 109/l) with S.E.M of hexaplicates.
* whole-body irradiation at 5 Gy causes significant decline of leukocyte and lymphocyte numbers (to almost zero values in the
case of lymphocytes), whereas local irradiation of the thorax area has insignificant effects on lymphocyte numbers in the
peripheral blood; numbers of granulocytes remained unaltered in both experimental settings over the interval considered
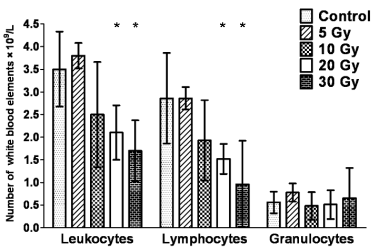
Fig. 2. Leukocyte differential count 24 h following the in vivo thoracic irradiation of rats. Columns show the number of
white blood elements (× 109/l) after exposure to different doses of ionizing radiation. The figure presents arithmetic means; error
bars show S.E.M of hexaplicates.
* indicates statistically significant values in the Student's t-test for leukocyte and lymphocyte counts after 20 and 30 Gy

Fig. 3. Microscopic detection of gammaH2AX in lymphocytes 1 h after in vivo thoracic irradiation of rats. The peripheral blood
lymphocytes were isolated from the heparinized peripheral blood of control and irradiated rats (doses 5-20 Gy) 1 h after
irradiation, fixed and gammaH2AX was detected by the phospho-specific antibody. The figure shows representative projections of
control (C) and irradiated samples obtained by fluorescence microscopy. Bright spots present H2AX foci, the lines surrounding
cell nuclei were drawn based on 4',6-diamidino-2-phenylindole (DAPI) counterstaining.
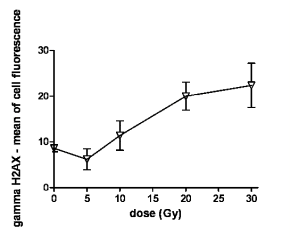
Fig. 4. Flow-cytometric quantification of gammaH2AX-FITC immunofluorescence in lymphocytes 1 h following the in vivo
irradiation. The lymphocytes were isolated from the heparinized peripheral blood of control and irradiated rats (doses of 5-30
Gy) 1 h after irradiation, gammaH2AX was detected immunocytochemically, by the antibody specific for H2AX phosphorylated on
serine 139 and simultaneously by analysis of the cellular DNA content. The mean of cellular fluorescence was measured by flow
cytometry for G0/G1 cells. Samples from 6 rats were evaluated in each group. The graph presents means of gammaH2AX-FITC
immunofluorescence depending on the gamma radiation dose. The results are statistically significant for doses of 20 and 30 Gy.
The figure presents arithmetic mean; error bars show S.E.M of hexaplicates.
RESULTS
Leukocyte differential count
Fig. 1 compares leukocyte differential counts in the
peripheral blood 24 h after whole-body and local
(thoracic) irradiation of rats. After whole-body
irradiation at 5 Gy, a massive decline of the
lymphocyte count was observed (almost to zero
values), whereas thoracic irradiation at the same dose
has only insignificant effect on the lymphocyte count
compared to the control non-irradiated group.
Fig. 2 demonstrates the leukocyte differential
count 24 hours after thoracic irradiation of rats at
doses of 5-30 Gy. Declines in leukocyte and
lymphocyte numbers were statistically significant
against the non-irradiated group from a dose of
20 Gy. Numbers of granulocytes were not signifi-cantly changed 24 h after thoracic irradiation.
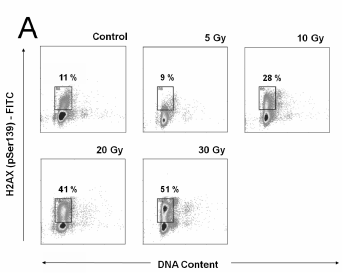
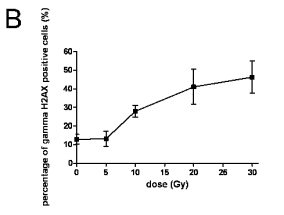
Fig. 5. A. Flow-cytometric detection of the gammaH2AX protein expression in lymphocytes 1 h after the in vivo thoracic
irradiation of rats (A) and gammaH2AX expression estimated according to gating analysis (B). The data are shown as
flow-cytometric bivariate histograms (dotplots) of the gammaH2AX-FITC immunofluorescence intensity on log scale (y axis) vs. DNA
content distributions (x axis). Representative dot-plots for 1 of 6 independently evaluated samples from 6 rats in each group are
shown. We used gating analysis to determine the IR-induced gammaH2AX expression. The gates were determined based on the
intrinsic ("programmed") gammaH2AX expression of controls. The percentage of cells falling into this gate was defined as the
percentage of gammaH2AX protein-positive cells. The graph of gammaH2AX expression estimated according to gating analysis described
in Fig. 5A presents percentage of gammaH2AX-positive cells versus dose of ionizing radiation. Samples from 6 rats were evaluated
in each group. The figure presents arithmetic means; error bars show S.E.M of hexaplicates.
Assessment of H2AX
By using flow cytometry as a quantitative method of
choice and fluorescence microscopy as a reference
method, we investigated the phosphorylation of
histone H2AX on serine 139 in lymphocytes of rats
exposed to thoracic irradiation at 5-30 Gy.
The images obtained by fluorescence microscopy
are shown in Fig. 3. We demonstrated increases in the
number of cells containing gammaH2AX foci with
increasing radiation doses (Controls 1.5%, 5 Gy 18%,
10 Gy 34%, 20 Gy 69%). However, even after the
highest dose (20 Gy), not all the cells contained
H2AX foci; this implies that not all the cells were
targeted by radiation. Individual gammaH2AX foci can be
identified in the cells isolated from rats exposed to
5 Gy, while after irradiation at 20 Gy the individual foci can no longer be distinguished and the positivity
is observed through the whole nucleus.
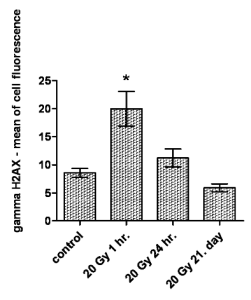
Fig. 6. Time-dependent kinetics of the gammaH2AX immunofluorescence intensity in peripheral blood lymphocytes of
thoracic-irradiated rats at a dose of 20 Gy. The figure presents arithmetic mean; error bars shows S.E.M of hexaplicates.
* statistically significant increase in the gammaH2AX expression 1 hour after irradiation and subsequent decline to control group values
within 24 hours, followed by additional decline under the level of control group on the 21st day after irradiation is shown
The increase in gammaH2AX and the proportion of cells
exposed to partial-body irradiation were further
quantified by flow cytometry. Fig. 4 shows a graph
representing the gammaH2AX quantification obtained by
flow cytometry. Specimens taken from 6 rats 1 hour
after thoracic irradiation were analysed in each group.
By using the flow-cytometric method, we observed
increases in the mean values of gammaH2AX
immunofluorescence in lymphocytes from the dose of
10 Gy; statistically significant at 20 and 30 Gy.
Since microscopy revealed non-homogeneous
gammaH2AX positivity among the lymphocytes, the flow
cytometry data were also studied but using a different
approach. Bivariate histograms (dotplots) of log
gammaH2AX-FITC immunofluorescence vs. DNA contents
were analysed to determine the percentage of gammaH2AX
positive cells. A gating analysis was used; the gate
was established to encompass the response of
IR-treated cells. The gates were estimated according
to the intrinsic gammaH2AX expression in the controls. The
representative flow cytometry dotplots are shown in
Fig. 5A. A graph, which shows the dose-dependence
of the percentage of gammaH2AX positive cells in the
whole set of the samples analysed (6 per group), is
shown in Fig. 5B. This method of data analysis also
verified the IR-induced increase in the gammaH2AX
expression after a dose of 10 Gy, which was
statistically significant (p0.05) at 20 and 30 Gy.
The time-dependent kinetics of the gammaH2AX
immunofluorescence intensity after thoracic
irradiation at a dose of 20 Gy is shown in Fig. 6. As
soon as 24 h after irradiation, the intensity of the
gammaH2AX immunofluorescence drops significantly
almost to the level of the control group. After 21
days, the level of gammaH2AX in the irradiated rats'
lymphocytes is lower than that in controls. This
indicates that lymphocytes with unrepaired IRIF are
quickly eliminated from the blood circulation.
DISCUSSION
The phosphorylation of histone H2AX on serine 139
is a very early DSB marker. However, the majority of
studies have been focused on gammaH2AX and IRIF
detection after in vitro irradiation of isolated cells. In
our previous work, we reported a dose-dependence of
the integral optical density (IOD) of phosphorylated
H2AX detected by confocal microscopy in in vitro
irradiated lymphocytes isolated from the peripheral
blood of human donors. The increase in gammaH2AX IOD
was dose-dependent up to 5 Gy and then reached a
plateau (Vilasova et al. 2008). Andrievski and
Wilkins (2009) compared the H2AX phosphorylation
in different lymphocyte subpopulations after in vitro
irradiation (0-10 Gy) of lymphocytes isolated from
the peripheral blood. The phosphorylation process
reached its maximum 1.5 h after irradiation and there
were only minimum differences between particular
lymphocyte subpopulations. Redon et al. (2009)
described a linear dependence on the increase in
gammaH2AX foci 30 min and 24 h after in vitro irradiation
of human lymphocytes. Due to the DNA repair, the
number of IRIF detected 24 h after irradiation was
lower by a factor of ten compared to that 30 min after
irradiation.
There are fewer data on H2AX phosphorylation
after in vivo irradiation. Lobrich et al. (2005)
evaluated changes in gammaH2AX 30 and 60 min after
irradiation of patients exposed to very low doses
during computerized tomography of the thorax and/or
abdomen. The average damage level in lymphocytes
from individuals exposed in vivo with DLP (defined
as the product of the dose deposit within the exposure
field and length of the body examined) of 150-1500
mGy/cm, achieved its maximum 30 min after
irradiation. The damage was repaired and foci
disappeared within 24 h after irradiation. At times of
30 and 60 min after irradiation, the average damage
level in lymphocytes from individuals exposed in vivo
to DLP of 1 000 mGy/cm is similar to that in
lymphocytes irradiated in vitro at a dose of 20 mGy.
Sak et al. (2007) studied the relationship between the
integral total body radiation dose (this dose was
estimated from the dose volume histogram of the
patient's body corrected for the proportion of the
body scanned by computerized tomography for 3D
treatment planning) and the number of gammaH2AX foci.
There was a strong linear correlation between the
mean number of gammaH2AX foci per lymphocyte in the
peripheral blood sample and integral total body
radiation dose (0.0089-0.354 Gy). The kinetics of the
in vivo gammaH2AX foci induction was studied by Redon
et al. (2010) in peripheral lymphocytes of rhesus
macaque after total-body gamma radiation exposure.
By the use of laser-scanning confocal microscopy,
they quantified mean values of gammaH2AX foci per cell
(fpc) in in vivo irradiated peripheral blood
lymphocytes depending on the time after the exposure
and radiation dose. A dose-dependent increase in the
gammaH2AX formation (fpc) was observed 0.3 day (doses
of 1-3.5 Gy) and 1, 2 and 4 days (doses of 1-8.5 Gy)
after irradiation. However, the numbers of fpc
quantified for each radiation dose substantially
decreased with increasing time after irradiation. The
numbers of persisting residual foci were proportional
to initial irradiation doses and statistically significant
(p0.05) responses were obtained up to the 1st day
after 1 Gy, 4 days after 3.5 and 6.5 Gy, and 14 days
after 8.5 Gy. In our study (Havelek et al. 2011) we
evaluated the dose dependence of the H2AX
phosphorylation in peripheral blood mononuclear
cells of rats exposed in vivo to whole-body irradiation at doses of 1-10 Gy. The data obtained by both
methods - the modified flow-cytometric method
published by Huang and Darzynkiewicz (Huang and
Darzynkiewicz 2006) and the microscopic detection
of gammaH2AX foci in individual cells - indicated a linear
dose dependence. While microscopic detection is
time-consuming and limited to hundreds of cells, the
flow-cytometric method makes possible objective and
relatively quick quantification of large numbers of
cells. It can be used as soon as 1 h after irradiation to
estimate the radiation dose received. Importantly,
unprocessed blood samples can be stored at 4 °C for
24 h and the results are not significantly affected
(Havelek et al. 2011). We can expect that the in vivo
gamma radiation-damaged lymphocytes in the
peripheral blood, which is sampled and ex vivo stored
(in 4 °C on ice) after irradiation, cannot be naturally
destroyed by the reticuloendothelial system of the
body. Storage at 4 °C also (on ice) inactivates
enzymes, which are phosphatases responsible for
gammaH2AX foci loss in this particular case.
In the work presented here, we described
dose-dependent increases in gammaH2AX in the
lymphocytes of rats irradiated in the thoracic area
occurring l h after the exposure to doses of 10 Gy and
above. Seven weeks after irradiation of the thoracic
area at doses of 15 and 20 Gy, based on histology, we
demonstrated the development of radiation
pneumonitis, an increase in the count of alveolar
neutrophils and alveolar septa thickening
(unpublished data). According to our knowledge, the
results presented here offer the first report describing
the gammaH2AX expression in lymphocytes isolated from
the peripheral blood of rats exposed to local
irradiation in the thoracic area. A maximum increase
in the mean of the gammaH2AX fluorescence intensity was
observed in a subpopulation of cells, which was
apparent from both microscopic and flow-cytometric
histograms. We can presume that the gammaH2AX positive
lymphocytes were present in the irradiated area
during the irradiation, while the gammaH2AX negative
ones were not present there that time. It is of
importance to note that only about 2% of all the
lymphocytes are present in the blood. The others
reside in other tissues, especially in the thymus,
lymph nodes, tonsils, intestines, spleen and bone
marrow. Eighty percent of all the lymphocytes
migrate between these tissues and the peripheral
blood, with an overall recirculation time of about
12 hours. It was estimated that the average time
period of the presence of a given lymphocyte in the
peripheral blood is 30 minutes, over which it travels
through the body (Westermann et al. 2001). This
implies that blood samples taken within one hour
after irradiation reflect the average dose given to the
peripheral blood (Rothkamm and Horn 2009).
In our experiments, both the mean value of the
gammaH2AX immunofluorescence in lymphocytes and the
percentage of gammaH2AX positive lymphocytes 1 h after
thoracic irradiation of rats exerted the same tendency
and increased significantly with increasing doses.
This was accompanied by dose-dependent
lymphocytopenia 24 hours after thoracic irradiation at
doses above 10 Gy. IR-induced lymphocytopenia is
considered an established haemopoietic mark of the
post-irradiation syndrome. In comparing
flow-cytometric and microscopic methods the
microscopic method is more sensitive in a range of
low doses (exposure of the thorax to 5 Gy) compared
to the method based on flow cytometry, since by the
microscopic method, it was possible to reveal foci in
18% of cells irradiated at 5 Gy, whereas flow
cytometry was not able to demonstrate any increase in
gammaH2AX and the lymphocyte count also did not
decrease in this group 24 h after irradiation compared
to controls. In contrast to this, the detection of
gammaH2AX is more precise after high doses (the foci
cannot be counted by the microscopic method any
more). From a long-term perspective, it would be of
interest to count persisting foci in irradiated
lymphocytes after the local exposure of the thorax.
ACKNOWLEDGEMENT
The authors would like to thank the Ministry of
Defence of Czech Republic (project
MO0FVZ0000501 and project OVUOFVZ200806)
for the financial support.
REFERENCES
Andrievski A, Wilkins RC. The response of gamma-H2AX in human lymphocytes and lymphocytes subsets measured in whole blood cultures. Int J Radiat Biol. 85: 369-376, 2009.
[CrossRef]
Bekker-Jensen S, Lukas C, Kitagawa R, Melander F, Kastan MB, Bartek J, Lukas J. Spatial organization of the mammalian genome surveillance machinery in response to DNA strand breaks. Cell Biol. 173: 195-206, 2006.
[CrossRef]
Blakely WF, Carr Z, Chin-May Chu, Dayal R, Fujimoto K, Hopmeir M. WHO 1st consultation on the development of a global biodosimetry laboratories network for radiation emergencies (BioDoseNet). Radiat Res. 171: 127-139, 2009.
[CrossRef]
Down JD. The nature and relevance of late lung pathology following localised irradiation of the thorax in mice and rats. Br J Cancer. 53 (Suppl. 7): 330-332, 1986.
Goans RE, Holloway EC, Berger ME, Ricks RC. Early dose assessment following severe radiation accidents. Health Phys. 72: 513-518, 1997.
Havelek R, Rezacova M, Sinkorova Z, Zarybnicka L, Blaha V, Vavrová J. Phosphorylation of histone H2AX as an indicator of received dose of gamma radiation after whole-body irradiation of rats. Acta Vet Brno. 80: 113-118, 2011.
Huang X, Darzynkiewicz Z. Cytometric assessment of histone H2AX phosphorylation: a reporter of DNA damage. Methods Mol Biol. 314: 73-80, 2006.
Jackson IL, Vujaskovic Z, Down JD. Revisiting strain-related differences in radiation sensitivity of the mouse lung: recognizing and avoiding the confounding effects of pleural effusions. Radiat Res. 173: 10-20, 2010.
[CrossRef]
Larsen DH, Poinsignon C, Gudjonsson T, Dinant C, Payne MR, Hari FJ, Danielsen JMR, Menard P, Sand JC, Stucki M, Lukas C, Bartek J, Andersen JS, Lukas J. The chromatin-remodeling factor CHD4 coordinates signaling and repair after DNA damage. J Cell Biol. 190: 731-740, 2010.
[CrossRef]
Lobrich M, Rief N, Kuhne M, Heckmann M, Fleckenstein J, Rube C, Uder M. In vivo formation and repair of DNA double-strand breaks after computed tomography examinations. Proc Natl Acad Sci USA. 102: 8984-8989, 2005.
[CrossRef]
Osterreicher J, Pejchal J, Skopek J, Mokry J, Vilasova Z, Psutka J, Vavrova J, Mazurova Y. Role of type II pneumocytes in pathogenesis of radiation pneumonitis: dose response of radiation-induced lung changes in the transient high vascular permeability period. Exp Toxicol Pathol. 56: 181-187, 2004.
Redon CE, Dickey JS, Bonner WM, Sedelnikova OA. Gamma-H2AX as a biomarker of DNA damage induced by ionizing radiation in human peripheral blood lymphocytes and artificial skin. Adv in Space Res. 43: 1171-1178, 2009.
[CrossRef]
Redon CE, Nakamura AJ, Gouliaeva K, Rahman A, Blakely WF, Bonner WM. The use of gamma-H2AX as a biodosimeter for total-body radiation exposure in non-human primates. PLoS ONE. 5: e15544, 2010.
[CrossRef]
Rogakou EP, Pilch DR, Orr AH, Ivanova VS, Bonner WM. DNA Double-stranded breaks induce histone H2AX phosphorylation on serine 139. J Biol Chem. 273: 5858-5868, 1998.
Rothkamm K, Horn S. GammaH2AX as protein biomarker for radiation exposure. Ann Ist Super Sanita. 45: 265-271, 2009.
Sak A, Grehl S, Erichsen P, Engelhard M, Grannass A, Levegrun S, Pottgen C, Groneberg M, Stuschke M. Gamma-H2AX foci formation in peripheral blood lymphocytes of tumor patients after local radiotherapy to different sites of the body: dependence on the dose-distribution, irradiated site and time from start of treatment. Int J Radiat Biol. 83: 639-652, 2007.
[CrossRef]
Travis EL. The sequence of histological changes in mouse lungs after single doses of x-rays. Int J Radiat Oncol Biol Phys. 6: 345-347, 1980.
Vavrova J, Filip S. Radiosensitivity of the Haemopoietic System (in Czech). Galen, Praha ISBN 80-7262-200-5, 99 p, 2002.
Vilasova Z, Rezacova M, Vavrova J, Tichy A, Vokurkova D, Zoelzer F, Rehakova Z, Osterreicher J, Lukasova E. Changes in phosphorylation of histone H2AX and p53 in response of peripheral blood lymphocytes to gamma irradiation. Acta Biochim Pol. 55: 381-390, 2008.
Westermann J, Engelhardt B, Hoffman JC. Migration of T cells in vivo: molecular mechanisms and clinical applications. Ann Intern Med. 135: 279-295, 2001.
|
BACK
|








