Journal of APPLIED BIOMEDICINE
ISSN 1214-0287 (on-line)
ISSN 1214-021X (printed)
Volume 9 (2011), No 4, p 173-183
DOI 10.2478/v10136-011-0013-4
Neurodegenerative diseases and neuroprotection: current views and prospects
Andre Nieoullon
Address: Andre Nieoullon, Institut de Biologie du Developpement de Marseille-Luminy (IBDML) CNRS, Aix-Marseille Universite, UMR6216, 13288 Marseille
cedex 9, France
andre.nieoullon@univmed.fr
Received 20th July 2011.
Revised 16th August 2011.
Published online 9th September 2011.
Full text article (pdf)
Summary
Key words
Introduction
From neurodegenerative diseases to proteinopathies
Some common characteristics of the neurodegenerative diseases
What about the causes of neurodegenerative diseases?
Further considerations on neuronal death mechanisms
The neuroprotection paradigm in neurodegenerative diseases
Conclusive remarks
References
SUMMARY
Most of the pathophysiological processes of neurodegenerative diseases share the aggregation of related proteins which is one of the hallmarks of
the degenerative processes. Recent advances in the knowledge of these proteinopathies show that the same protein could contribute to a number of
diseases, thus suggesting a common pathological process. If this is so, specific instances of the brain neuronal system targeted by protein
dysfunction could be a sign of a differential clinical expression rather than different pathological processes. This very stimulating view of the
neurodegenerative diseases based on physiopathology has led us to suggest that possible degenerative mechanisms may be shared by different diseases
although the causes of the disease itself still remain unclear. Since genetic forms of the degenerative diseases are rather rare, exploring the
involvement of genes is one current way of researching the degenerative process of the disease. It is has thus been speculated that idiopathic forms
of the diseases are related to close interactions between genetic and environmental factors; the genetic component being able to favour - or
perhaps, to protect against - the disease process. Because of the current view that the basic mechanism of cell death in degenerative diseases is
related to a rather limited number of processes in which oxidative stress could play a central role resulting in protein dysfunction and
aggregation, one can speculate that there are neuroprotective medicines soon to be proposed based on the active limitation of protein accumulation
in the brain.
KEY WORDS
neurodegenerative diseases; proteinopathies; Alzheimer's disease; Parkinson's disease; oxidative stress; proteasome; neuroprotection
INTRODUCTION
Although Alzheimer's disease is in the forefront of
our thinking about neurodegenerative disorders it
should not be forgotten that such diseases in general
form a very large proportion of the total number of
neurological diseases, and represent a very high
financial cost for society. A recent study (see
Andlin-Sobocki et al. 2005) has evaluated the total
cost of brain diseases per year, including direct and
indirect costs, in 28 countries in Europe at about
386 billion Euros for the year 2004. This represented
35% of the total burden of diseases affecting about
27% of the 465 million people who are suffering brain
diseases. If mental disorders are excluded from the
calculation the total cost of neurological diseases
including dementia could be about 146 billion Euros
per year and the total specific cost of the
neurodegenerative diseases could be as much as
72 billion Euros (Table 1). These diseases are found
in about 5% of the total number of patients suffering
brain diseases. They are characterized by more or less
selective neuronal degenerations inducing neuro-logical syndromes, and affect both sensory-motor
areas and cognitive functions.
FROM NEURODEGENERATIVE DISEASES TO PROTEINOPATHIES
Clinical semiology has contributed to the well
accepted classification of the neurodegenerative
diseases, the main representatives of which are
Alzheimer's disease and related dementia,
Parkinson's disease, amyotrophic lateral sclerosis
(ALS), fronto-temporal-dementia (FTD) and
Huntington's disease among many others. Recent
advances in genetics and molecular biology have led
us, however, to reconsider this clinical classification.
The presence of amyloid peptide accumulations in the
extracellular space, is considered a patho-physiological hallmark of Alzheimer's disease but
such indications in general have been recently
revisited, to take into account the fact that some of
these diseases are characterized by similar
intraneuronal accumulations of particular proteins
such as for example the tau protein (acting as a
microtubule associated protein). Such a special tau
protein accumulation thus contributes to the
delineation of a subgroup of neurodegenerative
diseases considered as tauopathies, including
Alzheimer's disease, cortico-basal degeneration,
supra nuclear progressive paralysis and certain forms
of FTD. Similarly, accumulations of -synuclein,
where the natural function of the protein is still
unknown, contribute to define the synucleinopathies
subgroup, which includes Parkinson's disease,
multisystematized atrophy and Lewy body dementia
(Ozawa et al. 2006). More recently a subcategory of
FTD was shown positive for another protein -
ubiquitin - but negative for tau protein accumulation.
These particular FTD, however, accumulate the
transcription factor TAR DNA-binding protein 43
(TDP-43), contributing to the definition of a new
class of proteinopathies including FTD associated to
ALS (Neumann et al. 2006, Josephs 2008).
These proposals (see Derkinderen 2009) are of
major importance, since, although they do not change
the clinical classifications, they are of considerable
heuristic value and contribute to the idea of possible
common pathophysiological processes, having very
different clinical expressions. In short, in considering
the similar conceptual approaches to these different
diseases one can speculate on the actual possibility of
future putative innovative treatments based on the
etiology of diseases which may apply to the entire
group of pathologies belonging to the same protein
entity (see Vajda 2002).
SOME COMMON CHARACTERISTICS OF
TH NEURODEGENERATIVE DISEASES
Neurodegenerative diseases have in common the very
progressivity of neuronal death, and this contributes
to the difficulty of establishing valuable animal
models of the diseases. In general, such models,
developed primarily using lesion experiments, do not
in fact reproduce the progressivity of the disease.
Unfortunately, the problem is not solved by genetic
modelling of the diseases based on selected gene
inactivation or over-expression in mice. Thus, the
pathophysiological mechanisms of the diseases in
general still remain largely unknown.
The progressivity of neuronal death has also led to
speculation on the very long prodromal phase of the
diseases, which asks key questions concerning early
diagnosis and treatment; a stage at which the clinical
signs of the disease are not expressed. The existence
of a prodromal phase of the diseases also contributes
to questions about mechanisms of the brain related to
neuroplasticity, which very efficiently compensate -
possibly for decades - for the clinical expression of
related brain lesions. It is thus claimed that the earlier
the treatment of the disease, the better it would be for
limitation of neuronal destruction and clinical
expression. Such a consideration emphasizes the
search for early events of the diseases and critical
putative biomarkers (see Hampel et al. 2010) as well
as stimulating research programs to promote
therapeutical approaches focused at the etiological
level leading to true neuroprotection against the
diseases.
In addition to the ethical problems linked to the
announcement to the patient of a heavy diagnosis in
the absence of curative treatment - such as for
example in familiar diseases of the Huntington's
disease type - the question of early diagnosis also
points to the difficulty clinicians have in relation to
differential diagnosis at a stage when clinical signs
are still limited. Such early diagnosis, however, is
absolutely necessary to further develop the evaluation
of the rather subtle first putative clinical signs of the
disease. Clinical evaluation and development of
cerebral brain imaging methods would further
contribute to early diagnosis of the neurodegenerative
diseases. Interestingly such early diagnosis also
involves the development of new biomarkers, the
validation of which is currently carried out to produce
an index of the follow up of developing brain lesions (Hampel et al. 2010). For example, in Alzheimer's
disease new measurements of CSF concentrations of
tau protein (native and/or phosphorylated forms)
and/or Abeta1-42 peptide are still possible, although
certain of these biomarkers could also be linked to
some other forms of the neurological diseases, which
question their actual specificity.
Table 1. Number of cases and cost per case and per year of neurodegenerative diseases in 28 selected European countries
(2004)*
|
Number of cases |
Cost per case/year |
Total cost/year | | Parkinson's disease |
1 160 000 |
7 500 Euros |
8.70 billion Euros | | Dementia |
4 890 000 |
11 000 Euros |
53.80 billion Euros | | Multiple sclerosis |
380 000 |
24 000 Euros |
9.12 billion Euros |
* from Andlin-Sobocki et al. (2005)
Another current characteristic of the neurodegenerative diseases is linked to the rather selective
systemic aspect of brain lesions, at least at the time
when the degenerative process is starting. Such
selectivity asks for the determination of the conditions
of the preferential vulnerability of the given neuronal
systems compared to others, and further suggests
possible therapeutic responses oriented toward these
particular neuronal systems when such conditions will
be determined. This question also constitutes an open
field for further investigation in the study of the
neurodegenerative diseases.
WHAT ABOUT THE CAUSES OF
NEURODEGENERATIVE DISEASES?
In the sporadic forms of the neurodegenerative
diseases, causative factors still remain largely
unknown. For Alzheimer's disease and possibly
Parkinson's disease, ageing is considered as a major
risk factor because of the increased incidence of the
diseases with age. In the case of Alzheimer's disease
the situation is clear, but in the other case such an
assessment is open to question since for a large
proportion of patients the starting point of Parkinson's
disease could be very early, and masked by a long
prodromic phase of the disease.
Some familiar forms of the diseases reveal a
genetic component but monogenic cases are very rare
and even exceptional (see Corti et al. 2010). This is
the case in early-onset forms of Alzheimer's disease
where the amyloid hypothesis is supported by
mutations of genes causing the disease, such as
mutations of gene encoding amyloid protein precursor
(chromosome 21) or presenilins (PS1 and PS2 located
on chromosomes 1 and 14, respectively) in rare
autosomic dominant forms (<5%). A similar situation
is found, for example, in autosomic dominant forms
of Parkinson's disease, with the gene encoding -synuclein (mutation PARK1 and PARK4) and LRRK2
(mutation PARK8) among 15 different identified
mutations. In some families, where FTD and ALS
cosegregate, mutations on one gene linked to
chromosome 19 could induce both clinical
phenotypes, which supports the concept of a common
mechanism, as revealed by new data on protein
accumulations (see Sleegers et al. 2010).
On the other hand, it has been speculated that, in
some patients, environmental factors can be involved
in the development of particular neurodegenerative
diseases (Cicchetti et al. 2009). For example,
compounds such as fungicides, pesticides, heavy
metals, substances derived from some addictives
drugs, or even a virus, could possibly play a role in
causing Parkinson's disease (see Moisan et al. 2011).
However epidemiologic studies have to be further
developed since present conclusions are in general
not sufficiently clear, therefore suggesting that sole
exposures to such environmental factors - even when
prolonged and intensive - are not usually sufficient to
induce the diseases (Dick 2006).
Interestingly, in relation to environmental factors
which could influence the occurrence of
neurodegenerative diseases, a new direction has
recently been taken focusing on stress. Intensive
chronic stress in humans was for example associated
with reduced hippocampal and right orbito-frontal
volumes (Gianaros et al. 2007), which may impact
cognitive and emotional functions. Consequently, the
durable altered activity of the hypothalamo-pituitary
axis could affect brain integrity. In experimental
studies, however, the most potent effects of stress are
observed when experiences occur in the early or even
prenatal phases of life. Indeed, stress in early life
appears to induce changes in adult behavior, such as
increased anxiety (see Marais et al. 2008).
Experiments using early maternal separation in
rodents show reduced hippocampal volume (Llorente
et al. 2009) and thickness of the dorsal prefrontal
cortex (Spivey et al. 2009), concomitant with
increased neuronal apoptosis in the hippocampus and
cortex. It has therefore been proposed that maternal
stress sensitizes the individual to later stress factors.
Thus, in the case of Parkinson's disease, it was
speculated that pathogenic events occurring during
early development may actually represent a source of
vulnerability to the later disease (see Barlow et al.
2007). In this respect maternal separation in rats was
shown to increase 6-hydroxydopamine-induced
behavioural impairment in the adult (Mabandla and
Russell 2010). So, early chronic stress could possibly
be a factor of vulnerability to Parkinson's disease and
possibly more generally of neurodegenerative
diseases in the adult, the mechanisms of which have
to be further investigated.
So, it is generally admitted that the sporadic cases
of neurodegenerative diseases - the vast majority in
fact - are linked to close critical interactions between
environmental and genetic factors. Consequently, it is
proposed that the differential expression of certain
genes or selected gene mutations will predispose to
the toxic action of environmental factors on specific
neuronal populations. Such a genetic susceptibility to
environmental factors would explain why certain
people develop the disease whereas others in the same
population do not when subjected to similar
environmental conditions. In the same way it can be
proposed that if certain people are more sensitive than
others to environmental conditions, then conversely
the apparent resistance to such factors could be linked
to specific genetic "neuroprotective" factors. There is
presently a lack of direct evidence for this, but we
know that the differential expression of epsilon2, epsilon3 or epsilon4
alleles of the apolipoprotein E (APOE) gene located
on chromosome 19 could either increase or decrease
the risk factor of developing Alzheimer's disease.
Indeed, in the CNS, APOE is primarily
synthesized and secreted by astrocytes and microglial
cells and contributes to the metabolism of cholesterol
and lipids. Experimentally the astrocytic expression
of human APOE3 or E4 has resulted in an
isoform-dependent effect on the Abeta peptide deposit
thus showing increased deposits in the case of APOE4
expression (Holtzman et al. 2000). Thus APOE4
appears as a true genetic risk factor since its
frequency increases in late-onset forms of
Alzheimer's disease. Conversely, the epsilon2 isoform may
represent a factor contributing to the reduction of the
risk of developing the disease by acting as a
"neuroprotective". In this context the over-expression
of the epsilon4 allele is a risk factor possibly interacting
with other genetic or non genetic factors to facilitate
the occurrence of Alzheimer's disease.
Other theories have been developed to explain the
occurrence of neurodegenerative diseases. Briefly,
3 groups of factors have been proposed. The first
group of theories involves endogenous factors
inducing a neuronal toxicity such as free radicals at
the origin of oxidative stress or the neurotransmitter
glutamate, which could destroy neurons by a process
called "excitotoxicity". The second group of theories
assumes that the neurodegenerative process could be
due to a lack of production or the action of more or
less specific energetic (mitochondrial defect) or
neurotrophic cellular factors; an impairment of
physiological retrograde axonal transport; an
alteration in cellular adhesion processes, or even to a
synaptic deficit in the neurotransmitter function or
synaptic metabolism such as glutamate inactivation
mechanisms. Finally, neurodegenerative processes
could also be linked to immunodeficiency
mechanisms such as in the case of auto-immune
pathologies which involve specific proteins exposed
to cellular surface, or to inflammatory processes.
Inflammatory processes, possibly involving
microglial cells are indeed presently thought to be of
primary importance in neurodegeneration such as in
Parkinson's disease (see Hirsch and Hunot 2009).
Last but not least, some other hypotheses of the
abiotrophic family have been proposed, suggesting
that neuronal degeneration involves an increase of
natural cellular death mechanisms or, in some cases,
alterations of the cell cycle.
It is therefore worth mentioning that if neuro-degenerative processes can be linking to such
different mechanisms, involving either/or genetic
components and external/internal signals, the
mechanisms of neuronal death could, conversely,
involve a considerably limited number of cellular
and/or molecular events. These events could represent
key elements as common effectors of cellular death in
neurodegenerative diseases. Such a consideration thus
refers to the above presented classification of
proteinopathies which may involve for each of them
several neurodegenerative processes suggesting that
putative common therapeutic solutions have to be
considered.
FURTHER CONSIDERATIONS ON
NEURONAL DEATH MECHANISMS
It is worth mentioning at the outset, that nowadays, in
spite of exceptional advances in cellular and
molecular biology, it is quite impossible to precisely
evaluate the contribution of apoptosis mechanisms in
neurodegenerative diseases. We can assume,
however, that such a mechanism is involved.
Apoptosis refers to an active process of cellular death
involving selective activation of a family of
cystein-proteases and associated proteins such as for
example the PARP protein, a protein polymerase
involved in DNA reparation processes, and numerous
other proteins such as those corresponding to the
caspase family (13 genes in human). Apoptosis,
therefore, can be viewed, at least partly, as an
organized and regulated cascade of gene activation
encoding cystein-proteases, the final issue of which is
endonuclease activation, inducing characteristic
structural changes in the cells. The apoptosis cascade,
however, was primarily described from physiological
data related to developmental processes contributing
to the elimination of over-numeric cells, which
depend, in the case of neurons, on cellular activity
and the sufficient availability of specific trophic
factors, and not from pathological processes.
Nevertheless, it can be mentioned that either the prion
protein or beta-amyloid peptide are active in the
promotion in vitro of DNA fragmentation similar to
that seen in a true apoptosis mechanism. Such
experimental data further suggest an involvement of
apoptosis in neuronal death related to
Creutzfeld-Jakob or Alzheimer's disease.
To suggest that apoptosis contributes as a sort of
"final common pathway" to neuronal death in many
neurodegenerative processes is consequently of great
heuristic value since apoptosis is subjected to
numerous genetic and epigenetic mechanisms
including environmental factors. From such a point of
view it is possible to imagine the intervention of
"apoptosis-inducing factors" which can be opposed to
the view of "apoptosis-inhibiting factors" which may
contribute to tumourisation and cancers. Further, we
can speculate that neuronal death in neurodegenerative diseases could actually be regulated. A
strategy could be proposed to promote a true
neuroprotection procedure balancing the
neurodegenerative process and contributing to
slowing and further ideally stopping the degenerative
disease.
As already mentioned, free radical production is
considered a main actor in the case of neuronal death
in relation to the oxidative stress theory. Numerous
experimental data support such a hypothesis. Indeed,
the reduction of molecular oxygen is a source of
superoxide derivatives, which is amplified by the
production of nitric oxide (NO) or calcium ions
contributing to deleterious cellular actions through
proteases, endonucleases and calpains activation. Iron
tissular concentrations as Fe2+ in combination with
oxygen peroxide further contribute to the production
of free radicals of the hydroxyl type (called the
'Fenton reaction') and may be involved in the
degenerative process of dopamine neurons in the case
of Parkinson's disease, because of the pro-oxidative
state of the substantia nigra pars compacta where the
neurons are located.
With reference to Parkinson's disease, it is worth
mentioning that oxidative stress and free radical
action are related to the increased activity of the
microglial cells present in the neuronal environment,
which could contribute to the promotion of oxidative
stress through an increase in the production of
cytokines and NO-synthase activation at the origin of
an inflammatory state. This is the focus of the work of
numerous research groups. In this respect the recent
identification of a lymphocyte penetration into the
substantia nigra studied in Parkinsonian patients (see
Brochard et al. 2009) further reinforces this proposal
and contributes to the justification for the use of
anti-inflammatory medications in Parkinson's
disease.
Oxidative stress can also be related to mito-chondrial functional impairment, possibly inducing a
decrease in complex I chain activity and cellular
metabolism (Kristian et al. 2011). Consequently,
oxidative phosphorylation could be reduced,
membrane potential of the mitochondria will be lost
and cytochrome C released into the cytoplasm, thus
further contributing to caspase activation. Such a
mitochondrial alteration could actually be involved in
the development of certain neurodegenerative
processes (Barsukova et al. 2011). For example, at
least 3 of the mutated proteins described in
Parkinson's disease (Parkin, DJ-1, and PINK1) are
located in the mitochondria, which indicates a
possible protective physiological role for
mitochondria against the establishment of the disease.
In recent years, research has also been focused on
the protein degradation process involving proteasome.
Such a process could represent another major possible
explanation for some degenerative diseases. Indeed,
ubiquitinylation is a known signal of protein
degradation through the proteasome machinery. Such
a process also depends on ATP and request sequential
activation of enzymes adding ubiquitin to substrate
proteins (Berger et al. 2006). In this case, enzymes
involved in desubiquitinylation processes
(hydrolases) further contribute to ubiquitin recycling,
which is a key step for addressing proteins to
proteasome degradation machinery (Ardley et al.
2004). Events contributing to the degradation of
proteins through proteasome activation are highly
sensitive to oxidative stress and free radicals. In
Parkinson's disease, for example, one of the enzymes
contributing to the cycle known as ubiquitin ligase 3
is assimilated to the parkin gene, the mutation of
which (PARK2 mutation) is associated with juvenile
Parkinson's disease (recessive autosomal
transmission). Similarly, the PARK8 mutation
(dominant autosomal transmission) impairs
leucin-rich repeat kinase (LRRK2; also called
dardarin) protein function and could impair protein
degradation through proteasome machinery and
contribute to alpha-synuclein deposits contributing
indirectly to the formation of Lewy bodies in
Parkinson's disease and, possibly, to hyperpho-sphorylated tau protein aggregates in Alzheimer's
disease (see Abou-Sleiman et al. 2006, Berger et al.
2010).
Altogether these processes could be interdependent. One can speculate that it is possible to link
in the same theory mitochondrial deficits, oxidative
stress and proteasome machinery alterations, and even
ubiquitin processing. Consequently, if one of these
pathways is altered, such a limited defect will impair
the others. In such conditions apoptose activation will
actually be the final and definitive consequence for
the cell.
THE NEUROPROTECTION PARADIGM IN
NEURODEGENERATIVE DISEASES
In the context of the neurodegenerative diseases it is
speculated that the administration of an
etiopathogenic treatment which interferes with the
developing pathogenic process, is able to slow and
ultimately stop the evolution of the disease (see
Djaldetti et al. 2003). Nowadays such a treatment for
neurodegenerative diseases is not likely to exist in
spite of the incredible number of attempts made in
this direction (Velly et al. 2003, Labrande et al.
2006). Clinical trials with many hundreds of different
compounds have been unsuccessful. Causes of such
failures are multiple but at the same time it is highly
paradoxical to consider that, conversely, preclinical
data are extremely encouraging. Such a difference in
trials could be primarily due to the unsatisfactory
character of animal models of the neurodegenerative
pathologies, which reflect only very partially the
clinical situation. Moreover, such discrepancies could
also be due to the actual difficulty clinicians have in
evaluating neuroprotective effects in general because
of very long lasting pathologies (see Akwa et al.
2005).
Although not fully satisfactory, the characte-rization of the mechanisms of neuronal death at
cellular and molecular levels has nevertheless led to
the proposition of new therapeutical strategies
involving putative neuroprotective agents able to
interfere and slow experimentally-induced neuronal
death. In such a way the neuroprotection procedure
aims at slowing the rate of neuronal death through
interaction with the pathological process and
furthering the evolution of clinical signs of the
disease. The concept of a disease modifier was then
introduced in this way, based on clinical improvement
in patients. At the moment the concept of a disease
modifier is more likely to be based on a mechanistic
approach to the disease considered at cellular and
molecular level than on a true neuroprotection process
(see Citron 2004, Akwa et al. 2005).
If the different putative mechanisms of neuronal
death here discussed are considered, one can
speculate that the most popular approach to
neuroprotection nowadays would be to try to reduce
the oxidative stress at cellular level using anti-oxidant
substances. In such a way numerous compounds have
been proposed (vitamins A, C, E, polyphenols such as
resveratrol or even baicalin or baicalein, Q
co-enzyme, etc.) but clinical trials do not show any
real improvement. Other strategies are based on
oestrogen supplementation (see Tiwari-Woodruff et
al. 2007), administration of anti-inflammatory drugs,
calcium-stabilizing compounds or stimulators of the
energy production of the cell. Altogether these
compounds, directly or indirectly are thought to
decrease the free radical oxygen species and thus
oxidative stress. Interestingly, dietary restriction,
phytochemicals (curcumin derivatives; flavonoids)
and dietary lipids are also thought to act in the same
way.
Further, agonism of the peroxisome proliferator
receptor-gamma (PPAR) may have therapeutic
interest for limiting oxidative stress and neuroinflammation in Parkinson's disease (Hunter and Bing
2007), where evidence has been found of an
inflammation process in the substantia nigra of
patients, thus corroborating data in animal models
which further emphasize the contribution of glial cells
and peripheral immune cells. Consequently down-regulating these inflammatory processes could be of
major importance for slowing the degenerative
mechanisms (see Hirsch and Hunot 2009).
Ultimately, since genetic studies of the ageing
process in animal models - primarily drosophila or
the nematode Coenorhabditis elegans - have
produced evidence of different sets of mutations
related to an increased life-span for these animals, it
can be speculated that inducing such gene mutations
could contribute to increasing the resistance to
diseases in general and to neurodegenerative diseases
in particular. Interestingly it is worth mentioning that
most of these positive mutations (age-1, daf-2, clk-1,
old-1, etc.) result in decreasing oxidative stress and/or
slowing cell metabolism (Friedman and Johnson
1988). For example, age-1 encodes for a repressive
factor of superoxide dismutase (SOD) expression and
consequently the gene mutation results in increased
resistance against the reactive oxygen species. Thus,
it can be speculated that, in future, inducing such
mutations in homolog human genes is a way to
increase the resistance to the neurodegenerative
diseases related to oxidative stress.
Apoptose inhibitors such as Bcl2 proteins,
compounds acting on caspases, etc. have also been
thought to reduce the impact of neurodegenerative
diseases. Putative strategies could also involve
modulation of NF-kappaB signalling, which exerts a
putative anti-apoptotic influence. Moreover, other
putative neuroprotective strategies have been
proposed, based on stimulation by cells of the
production of neurotrophic factors, including physical
exercise, but also cellular and gene therapies.
Interestingly, one of these factors, the brain derived
neuronal factor (BDNF), has been recently
extensively studied and it has been speculated that
BDNF could actually have therapeutic interest (see
Nagahara and Tuszynski 2011). However, expe-riments recently performed in an experimental model
of Parkinson's disease using BDNF gene delivery do
not verify its positive contribution (Decressac et al.
2011). Nevertheless, one has to consider that BDNF
is not the sole candidate as a possible contributor to
brain homeostasis since more than 50 different
trophic factors such as GDNF or NGF, for example,
have been identified in the brain. Moreover, such a
result does not mean that BDNF gene delivery will
not be efficient in another neurodegenerative disease
although clinical trials have also failed for ALS (see
Nagahara and Tuszynski 2011). Interestingly, the
peptide mixture cerebrolysin, which exhibits
neurotrophic effects, was shown in the animal model
to reduce Abeta production and deposit (Rockenstein et
al. 2006).
Compounds acting to reduce glutamatergic
transmission could also be of interest regarding the
possible role of excitotoxicity in neurodegenerative
diseases. Animal models provide evidence of a
possible positive influence of compounds acting on
the glutamatergic receptor of the mGluR3 subtypes,
which are known to decrease glutamate release and of
a possible interest in cannabinoid agents acting as
CB1 receptor antagonists. But in this last case, data
are sometimes controversial in the literature.
Regarding a form of possible pre-disposition to
neurodegenerative diseases due to early events in the
life as suggested for Parkinson's disease (see
Mabandla and Russell 2010) the question thus arises
as to whether potential interventions which may
prevent chronic intense stress consequences in
juveniles, might provide some forms of neuroprotection strategies.
Last but not least, a therapeutical approach based
on immunotherapy (active or passive immunotherapy)
such as for example in Alzheimer's disease with a
view to reducing brain amyloid deposits, has been
investigated, as has also the use of amyloid-beta
modulating agents such as beta- or gamma-secretase inhibitors
(such as CHF5074) or amyloid beta-degrading agents
such as neprilysin (Meilandt et al. 2009), as well as
the administration of statins to limit cholesterol
deposits (Sierra et al. 2011) or beta-sheet breakers.
Similarly, the ubiquitin-proteasome system could
obviously represent a new target for developing novel
therapeutics not only for neurodegenerative diseases
but also immune-inflammatory disorders or even
infectious diseases (see Bedford et al. 2011). The
stimulation of cell survival pathways involving for
example Akt signalling, PACAP stimulation (Reglodi
et al. 2011), PARP modulation, NF-B or ERK
inhibition, could also represent an alternative to
apoptose inhibition. In the case of Parkinson's disease
one can further speculate that LRRK2 induced-
inhibition or induced-expression of the parkin, PINK1
or DJ-1, for example, could also contribute to the
protection of the dopaminergic neurons.
Modulation of neuronal excitability through
agents modulating Na+/Ca2+ transporters or ions
chelators such as desferrioxamine as well as a
different subset of neuropeptides like cortexin or
cortagen should also be of interest in the protection of
the brain against neurodegenerative diseases.
An idea recently developed is that physical
exercise could be one of the factors reducing the risk
of developing neurodegenerative disease. Of course
the demonstration of such assumption in humans is
rather difficult but convergent data have been
obtained from animal studies. Speculation on possible
mechanisms proposes that such activity will increase
the brain expression of trophic factors. Interestingly
such a concept was extended to protection against
cognitive decline based on the fact that certain
epidemiologic studies have emphasized a decreased
incidence of Alzheimer's disease in individuals who
have intense intellectual activity, good educational
and rather high cultural levels. So, the concept of "cognitive reserve" has been suggested, to represent
a protective factor against dementia as well as
physical activity.
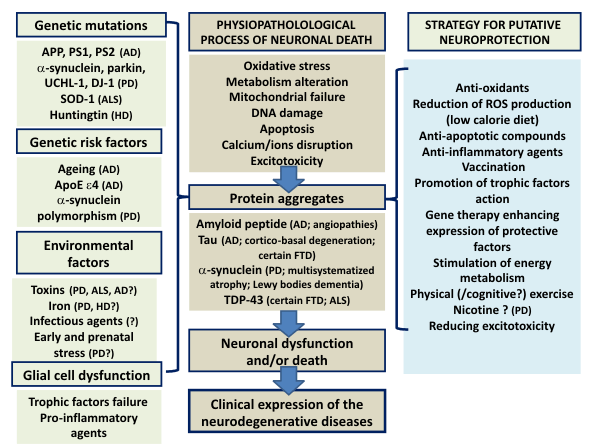
Fig. 1. Recapitulation of the processes which could be involved in the neurodegenerative diseases including genetic causal
mutations and genetic risk factors possibly interfering with environmental factors to cause the diseases (first column). The
second column presents possible mechanisms leading to protein aggregates and further to neuronal death, which contributes to
clinical expression in neurodegenerative diseases. The last column presents putative strategies for active neuroprotection (AD:
Alzheimer's disease; PD: Parkinson's disease; HD: Huntington's disease; ALS: amyotrophic lateral sclerosis; FTD:
fronto-temporal dementia; TDP-43: TAR DNA-binding protein 43; ROS: reactive oxygen species).
Whatever strategy has been developed, however,
at present the results are rather disappointing, except
for riluzole, an anti-excitatory compound which was
shown to be effective in ALS although the effects can
be considered as limited. Does it mean that we are
wrong and that we have to stop the search for putative
neuroprotective drugs? The answer is of course
certainly not. However, data could indicate that the
concept has to be investigated in a less mechanistic
direction or, alternatively, that we have to think in a
more pertinent way in order to appreciate the
neuroprotective effects in the clinic.
CONCLUSIVE REMARKS
In neuroscientific research, the field of neuro-degenerative diseases is one of the most active in
respect of both medical and associated social issues.
Recent advances in the basic knowledge of such
diseases have led to a re-evaluation of the
pathological processes and to speculation about new
therapeutical approaches (Fig. 1). These new
therapies will include strategies based on better
knowledge of the etiopathological processes and on
more effective neuroprotection. Such mechanistic
approaches to the diseases are still probably too
global to be fully efficient in the short term. But we
are fully convinced that new advances in the
knowledge of the physiopathological mechanisms of
the diseases including at molecular and genetic levels
will further contribute to new therapeutic
developments (see Nieoullon 2004). Research in this
field is very active. Indeed, in the single field of
Alzheimer's disease and related dementias, in 2010
nearly 100 medicines were being developed by
biopharmaceutical research, but we are still waiting
for definitive positive results. This is the reason why
the research efforts from state governments and the
EU, together with the pharmaceutical industry, have
to be reinforced, especially because, if we look at
neurodegenerative diseases at least partly as
proteinopathies it can be further speculated that
curing one of the diseases could possibly contribute
to a cure for the entire group of diseases related to a
given proteinopathy. In this respect many biotech
companies actively collaborate for example in the
development of novel therapies based on the
production of antibodies to modify the misfolding of
proteins in Parkinson's and Alzheimer's diseases.
REFERENCES
Abou-Sleiman PM, Hanna MG, Wood NW. Genetic association studies of complex neurological diseases. J Neurol Neurosurg Psychiatry. 77: 1302-1304, 2006.
[CrossRef]
[PubMed]
Akwa Y, Allain H, Bentue-Ferrer D, Berr C, Bordet R, Geerts H, Nieoullon A, Onteniente B, Vercelletto M. Neuroprotection and neurodegenerative diseases: from biology to clinical practice. Alzheimer Dis Assoc Disord. 19: 226-239, 2005.
[CrossRef]
Andlin-Sobocki P, Jonsson B, Wittchen H-U, Olesen J. Costs of disorders of the brain in Europe. Eur J Neurol. 12 (Suppl. 1): 1-27, 2005.
[CrossRef]
[PubMed]
Ardley HC, Scott GB, Rose SA, Tan NG, Robinson PA. UCH-L1 aggresome formation in response to proteasome impairment indicates a role in inclusion formation in Parkinson's disease. J Neurochem. 90: 379-391, 2004.
[CrossRef]
[PubMed]
Barlow BK, Cory-Slechta DA, Richfield EK, Thiruchelvam M. The gestational environment and Parkinson's disease: evidence for neurodevelopmental origins of a neurodegenerative disorder. Reprod Toxicol. 23: 457-470, 2007.
[CrossRef]
[PubMed]
Barsukova AG, Bourdette D, Forte M. Mitochondrial calcium and its regulation in neurodegeneration induced by oxidative stress. Eur J Neurosci. 10: 437-447, 2011.
[CrossRef]
[PubMed]
Bedford L, Lowe J, Dick LR, Mayer RJ, Brownell JE. Ubiquitin-like protein conjugation and the ubiquitin-proteasome system as drug targets. Nature Rev Drug Discovery. 10: 29-46, 2011.
[CrossRef]
[PubMed]
Berger Z, Davies JE, Luo S, Pasco MY, Majoul I, O'Kane CJ, Rubinsztein DC. Deleterious and protective properties of an aggregate-prone protein with a polyalanine expansion. Hum Mol Genet. 15: 453-465, 2006.
[CrossRef]
[PubMed]
Berger Z, Smith KA, Lavole MJ. Membrane localization of LRRK2 is associated with increased formation of the highly active LRRK2 dimer and changes in its phosphorylation. Biochemistry. 49: 5511-5523, 2010.
[CrossRef]
Brochard V, Combadiere B, Prigent A, Laouar Y, Perrin A, Beray-Berthat V, Bonduelle O, Alvarez-Fischer D, Callebert J, Launay JM, Duyckaerts C, Flavell RA et al. Infiltration of CD4+ lymphocytes into the brain contributes to neurodegeneration in a mouse model of Parkinson's disease. J Clin Invest. 119: 182-192, 2009.
[CrossRef]
[PubMed]
Cicchetti F, Drouin-Ouellet J, Gross RE. Environmental toxins and Parkinson's disease: what have we learned from pesticide-induced animal models? Trends in Pharmacol Sci. 30: 475-483, 2009.
[CrossRef]
[PubMed]
Citron M. Strategies for disease modification in Alzheimer's disease. Nature Rev Neurosci. 5: 677-685, 2004.
[CrossRef]
[PubMed]
Corti O, Fournier M, Brice A. Neurodegeneration in Parkinson's disease: genetics enlightens physiopathology. J Neural Transm Suppl. 73: 215-221, 2009.
[PubMed]
Decressac M, Ulusoy A, Mattsson B, Georgievska B, Romero-Ramos M, Kirik D, Bjorklund A. GDNF fails to exert neuroprotection in a rat (alpha)-synuclein model of Parkinson's disease. Brain. 134: 2302-2311, 2011.
[CrossRef]
[PubMed]
Derkinderen P. Classification of neurodegenerative diseases: things are getting complicated (French). La Lettre du Neurologue. 13: 68, 2009.
Dick FD. Parkinson's disease and pesticide exposures. Br Med Bull. 79-80: 219-231, 2006.
[CrossRef]
[PubMed]
Djaldetti R, Lev N, Melamed E. Neuroprotection in progressive brain disorders. Isr Med Assoc J. 5: 576-580, 2003.
[PubMed]
Friedman DB, Johnson TE. A mutation in the age-1 gene in Caenorhabditis elegans lengthens life and reduces hermaphrodite fertility. Genetics. 118: 75-86, 1988.
[PubMed]
Gianaros PJ, Jennings JR, Sheu LK, Greer PJ, Kuller LH, Matthews KA. Prospective reports of chronic life stress predict decreased grey matter volume in the hippocampus. Neuroimage. 35: 795-803, 2007.
[CrossRef]
[PubMed]
Hampel H, Franck R, Broich K, Teipel SJ, Katz RG, Hardy J, Herholz K, Bokde ALW, Jessen F, Hoessler YC, Sanhai WR, Zetterberg H et al. Biomarkers for Alzheimer's disease: academic, industry and regulatory perspectives. Nature Rev Drug Discov. 9: 560-574, 2010.
[CrossRef]
[PubMed]
Hirsch EC, Hunot S. Neuroinflammation in Parkinson's disease: a target for neuroprotection? Lancet Neurol. 8: 382-397, 2009.
[CrossRef]
Holtzman DM, Bales KR, Tenkova T, Fagan AM, Parsadanian M, Sartorius LJ, Mackey B, Olney J, McKeel D, Wozniak D, Paul SM. Apolipoprotein E isoform-dependent amyloid deposition and neuritic degeneration in a mouse model of Alzheimer's disease. Proc Natl Acad Sci USA. 97: 2892-2897, 2000.
[CrossRef]
[PubMed]
Hunter RL, Bing G. Agonism of peroxisome proliferator receptor-gamma may have therapeutic potential for neuroinflammation and Parkinson's disease. Current Neuropharmacology. 5: 35-46, 2007.
[CrossRef]
Josephs KA. Frontotemporal dementia and related disorders: deciphering the enigma. Ann Neurol. 64: 4-14, 2008.
[CrossRef]
[PubMed]
Kristian T, Balan I, Schuh R, Onken M. Mitochondrial dysfunction and nicotinamide dinucleotide catabolism as mechanisms of cell death and promising targets for neuroprotection. J Neurosci Res. 10: 1002-1016, 2011.
[CrossRef]
Labrande C, Velly L, Canolle B, Guillet B, Masmejean F, Nieoullon A, Pisano P. Neuroprotecive effects of tacrolimus (FK506) in a model of ischemic cortical cell cultures: role of glutamate uptake and FK506 binding protein 12KDa. Neuroscience. 137: 231-239, 2006.
[CrossRef]
[PubMed]
Llorente R, Gallardo ML, Berzal AL, Prada C, Garcia-Segura LM, Viveros MP. Early maternal deprivation in rats induces gender-dependent effects on developing hippocampal and cerebellar cells. Int J Dev Neurosci. 27: 233-241, 2009.
[CrossRef]
[PubMed]
Mabandla MV, Russell VA. Voluntary exercise reduces the neurotoxic effects of 6-hydroxydopamine in maternally separated rats. Behav Brain Res. 211: 16-22, 2010.
[CrossRef]
[PubMed]
Marais L, Van Rensburg SJ, Van Zyl JM, Stein DJ, Daniels WMU. Maternal separation of rat pups increases the risk of developing depressive-like behavior after subsequent chronic stress by altering corticosterone and neurotrophin levels in the hippocampus. Neurosci Res. 61: 106-112, 2008.
[CrossRef]
[PubMed]
Meilandt WJ, Cisse M, Ho K, Esposito LA, Scearce-Levie K, Cheng IH, Yu GQ, Mucke L. Neprilysin overexpression inhibits plaque formation but fails to reduce pathogenic Abeta oligomers and associated cognitive deficits in human amyloid precursor protein transgenic mice. J Neurosci. 18: 1977-1986, 2009.
[CrossRef]
[PubMed]
Moisan F, Spinosi J, Dupupet JL, Delabre L, Mazurie JL, Goldberg M, Imbernon E, Tzourio C, Elbaz A. The relation between type of farming and prevalence of Parkinson's disease agricultural workers in five French districts. Mov Dis. 26: 271-279, 2011.
[CrossRef]
Nagahara AH, Tuszynski MH. Potential uses of BDNF in neurological and psychiatric disorders. Nature Rev Drug Discovery. 10: 209-219, 2011.
[CrossRef]
[PubMed]
Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT, Bruce J, Schuck T, Grossman M, Clark CM, McCluskey LF, Miller BL et al. Ubiquinated TDP-43 in fronto-temporal lobar degeneration and amyotrophic lateral sclerosis. Science. 314: 130-133, 2006.
[CrossRef]
[PubMed]
Nieoullon A. Alzheimer's disease: neurobiological advances supporting proposals for new therapeutical approaches. J Appl Biomed. 2: 123-130, 2004.
[JAB]
Ozawa T, Healy DG, Abou-Sleiman PM, Ahmadi KR, Quinn N, Lees AJ, Shaw K, Wullner U, Berciano J, Moller JC, Kamm C, Burk K et al. The alpha-synuclein gene in multiple system atrophy. J Neurol Neurosurg Psychiatry. 77: 464-467, 2006.
[CrossRef]
[PubMed]
Reglodi D, Kiss P, Lubics A, Tamas A. Review on the protective effects of PACAP in models of neurodegenerative diseases in vitro and in vivo. Curr Pharm Des. 17: 962-972, 2011.
[CrossRef]
[PubMed]
Rockenstein E, Torrance M, Mante M, Adame A, Paulino A, Rose JB, Crews L, Moessler H, Masliah E. Cerebrolysin decreases amyloid-beta production by regulating amyloid protein precursor maturation in a transgenic model of Alzheimer's disease. J Neurosci Res. 15: 1252-1261, 2006.
[CrossRef]
[PubMed]
Sierra S, Ramos MC, Molina P, Esteo C, Vazquez JA, Burgos JS. Statins as neuroprotectants: a comparative in vitro study of lipophilicity, blood-brain-barrier penetration, lowering of brain cholesterol, and decrease of neuron cell death. J Alzheimer Dis. 23: 307-318, 2011.
[CrossRef]
[PubMed]
Sleegers K, Cruts M, Van Broeckhoven C. Molecular pathways of frontotemporal degeneration. Ann Rev Neurosci. 33: 71-88, 2010.
[CrossRef]
[PubMed]
Spivey JM, Shumake J, Colorado RA, Conejo-Jimenez N, Gonzales-Pardo H, Gonzales-Lima F. Adolescent female rats are more resistant than males to the effects of early stress on the prefrontal cortex and impulsive behavior. Dev Psychobiol. 51: 277-288, 2009.
[CrossRef]
[PubMed]
Tiwari-Woodruff S, Morales LB, Lee R, Voskuhl RR. Differential neuroprotective and anti-inflammatory effects of estrogen receptor (ER) alpha and ERbeta ligand treatment. Proc Natl Acad Sci USA. 104: 14813-14818, 2007.
[CrossRef]
[PubMed]
Vajda FJ. Neuroprotection and neurodegenerative disease. J Clin Neurosci. 9: 4-8, 2002.
[CrossRef]
[PubMed]
Velly L, Guillet B, Masmejean F, Nieoullon A, Bruder N, Gouin FM, Pisano P. Neuroprotective effects of propofol in a model of ischemia cortical cell cultures. Role of glutamate and its transporters. Anesthesiology. 99: 368-375, 2003.
[CrossRef]
[PubMed]
|
BACK
|


