Journal of APPLIED BIOMEDICINE
ISSN 1214-0287 (on-line)
ISSN 1214-021X (printed)
Volume 9 (2011), No 4, p 185-196
DOI 10.2478/v10136-011-0003-6
Alzheimer’s disease and related neurodegenerative disorders: implication and counteracting of melatonin
Miroslav Pohanka
Address: Miroslav Pohanka, Faculty of Military Health Sciences, University of Defense, Trebesska 1575, 500 01 Hradec Kralove, Czech Republic
miroslav.pohanka@gmail.com
Received 21st January 2011.
Revised 16th February 2011.
Published online 7th April 2011.
Full text article (pdf)
Summary
Key words
Introduction
Alzeimer's disease molecular pathogenesis
Alzeimer's disease and oxidative stress
Current options for Alzeimer's disease treatment
Action if antioxidants in pathogenesis of Alzeimer's disease
Melatonin biological effects
Melatonin potential for Alzeimer's disease treatment
Conclusions and expectations for the future
Acknowledgement
References
SUMMARY
Age related neurodegenerative disorders are becoming a serious public health problem. Alzheimer’s disease (AD) is a progressive disease
pathologically recognizable by deposition of neurofibrillary tangles and amyloid plaques. Oxidative stress probably plays a pivotal role in AD, but
despite expectations, antioxidants such as vitamin E, vitamin C, beta carotene, and flavonoids have failed as effective prophylaxis and/or
treatment. Melatonin, a hormone controlling circadian rhythm, is a potent terminal antioxidant. In vitro tests and animal models have
established that the application of melatonin could be beneficial for the amelioration of AD progression. Unfortunately, melatonin effects in human
beings are not well understood and a lot of work has still to be done. The review summarizes the basic facts about melatonin and its prospects as a
treatment for AD using its hormonal and antioxidant properties.
KEY WORDS
Alzheimer’s disease; amyloid plaques; neurofibrillary tangles; tau; amyloid beta; oxidative stress; melatonin; antioxidant
INTRODUCTION
Alzheimer's disease (AD) is a neurodegenerative
disorder with a not well understood aetiology. AD has
become not only an ethical problem in public health
care but it also an increasing item in care costs
(Jonsson and Wimo 2009). Schumock (1998), for
example, estimated the costs of AD treatment at the
end of the 1990s, and calculated that the total cost per
patient in the United States was 195, 000 USD,
including nursing, drugs and family out-of-pocket
expenses.
The aetiology of AD has not yet been established.
It is recognized that amyloid beta and
hyperphosphorylated tau protein (HPT) accumulates
before the development of manifest AD (Leinonen et
al. 2010), but the precise diagnosis of AD is not
simple. The Mini-Mental State Examination (MMSE)
cognitive test, lasting approximately 45 minutes, is a
simple option for fast diagnosis of dementias
including AD (Wilson et al. 2002), because AD, as
well as other dementias, can be accompanied by
cognitive dysfunction, by apathy, aggression,
irritability, and aberrant motor behavior; although
anxiety and apathy seem to be more typical of AD
than of the other dementias (Wetzels et al. 2010).
As will be discussed later, AD is closely
associated with the impairment of the electron
transport chain in mitochondria and the consequent
oxidative stress (Pickrell et al. 2009). The application
of an antioxidant is hypothesized as a promising
treatment for AD (Flaherty et al. 2010). Melatonin is
a potent antioxidant and endogenous hormone, and,
when administered exogenously, it can act as a potent
protective drug against oxidative stress related
disorder and intoxications (Pohanka et al. 2011a). The
present review considers the implication of oxidative
stress in AD, the potency of antioxidant treatment,
gives a summary of the known facts and an estimation
of promising ways to use melatonin as a drug for
ameliorating AD pathogenesis.
ALZHEIMER'S DISEASE MOLECULAR
PATHOGENESIS
Two major molecular mechanisms are related to AD
pathology: amyloid beta deposition in the form of
amyloid plaques and the creation of HPT forming
neurofibrillary tangles. Amyloid beta is cleaved from
the amyloid precursor protein (APP), but neither the
physiological role of APP nor the mechanism of
cleavage of the 42 amino acids long amyloid beta
fragment (1-42) is fully understood. APP splitting is
carried out by three secretases: alpha, beta, and gamma. Fragments
provided by alpha-secretase are non-toxic and are not
further modified by beta- and gamma-secretases into
potentially dangerous forms (Cole and Vassar 2007).
Neurotoxic amyloid beta is created in two steps. In
the first step, APP is cleaved by beta-secretase (known
also as an aspartic protease BACE 1 or memapsin 2).
In the second step, gamma-secretase containing the
transmembrane protein presenilin finalizes the
production of amyloid fragments (Coen and Annaert
2010). Amyloid beta peptides of different lengths can
appear; typically 39-43 amino acids long (Kadlick et
al. 2004). The most neurotoxic is the amyloid beta
consisting of 42 amino acids (Jan et al. 2011a), which
dominates the shorter amyloid peptides in AD
patients (Kuperstein et al. 2010). Moreover, it is the
most hydrophobic and fibrillogenic form of the
cleaved fragments and it can simply form amyloid
plaques in neurons (Murphy and LeVine 2010).
HPT is the second hallmark of damaged neurons
in AD patients, and this pathogenesis is based on the
deposit of neurofibrillar tangles inside the neurons.
The origin of the tau is in the cytoskeleton where it
stabilizes microtubules (Kao et al. 2010), becomes
hyperphosphorylated due to not well understand
mechanisms and can cause dementia diseases called
tauopathy. Beside tauopathy in AD patients, tau is
also implicated in the pathogenesis of Parkinson's
disease (Lei et al. 2010). HPT contains phosphates
bound via GSK-3beta kinase into Ser199, Ser202, Ser
396, and Ser 404 (Cai et al. 2011).
Two major molecular changes relate to AD. The
link between neurofibrillary tangles and amyloid
plaques is not well recognized, but the deposition of
amyloid plaques probably starts before the formation
of neurofibrillary tangles (Zheng et al. 2002). The
whole pathogenesis is also tightly connected to the
immune system and neuroinflammation probably
plays a crucial role in the development of AD (Casoli
et al. 2010). Although AD is not specific to a
particular region, the cerebral cortex and
hippocampus are the most damaged regions (Raji et
al. 2009).
ALZHEIMER'S DISEASE AND OXIDATIVE
STRESS
There are several theories of senescence.
Unfortunately, a definite answer to the question of
why and how ageing appears has not been
established. Two theories relating to AD, seem to be
the most plausible: firstly, ageing is the result of the
chronic impact of reactive oxygen species, and
secondly, resistance to oxidative stress is decreased
due to some endogenous and/or exogenous effectors
(Gilca et al. 2007). Oxidative insult probably triggers
or augments AD pathology. On the other hand, the
aetiology of many diseases and tissue damage may
relate to oxidative stress and some authors suggest
that reactive oxygen species are only a consequence
and not a primary cause in these situations (Juranek
and Bezek 2005).
Amyloid beta (1-42) toxic impact is not based
only on the deposition of amyloid plaques but also on
the adverse effects of redox reactions. Met 35 is
responsible for the toxic properties of amyloid beta as
it is associated with the generation of oxidative stress
and the oxidative modification of macromolecules
(Butterfield and Kanski 2002). Replacement or
oxidation of Met 35 leads to the abolition or reduction
of amyloid beta (1-42) toxicity (Clementi et al. 2006).
The molecular mechanism of the pro-oxidant
activities of amyloid beta (1-42) is not clear. It can
reduce CuII+ to CuI+ and thus trigger a Fenton reaction
(Boyd-Kimball et al. 2004), and it can also initiate
cytochrome c release from the mitochondria and
activation of apoptotic cascades. Substitution of Met
35 by norleucine abrogates the apoptotic cascade as
proven on PC12 cell lines (Clementi and Misiti 2005).
Cell lines affected by amyloid beta (1-42) and its
fragments suffer from oxidative insult via the
excessive production of nitric oxide, superoxide, and
hydrogen peroxide as well as their reaction products
such as peroxynitrite (Ill-Raga et al. 2010).
Trans-4-hydroxy-2-hexenal is proven reactive product
of the degradation of lipid membranes in the presence
of amyloid beta (1-42) in neuronal cell lines or the
brains (Butterfield and Lauderback 2002). The
implication of amyloid beta (1-42) in oxidative stress
is also confirmed by the fact that antioxidants such as
vitamin E prevent amyloid beta (1-42) induced
oxidative stress in model animals (Butterfield 2002).
The link between tau respective HPT and
oxidative stress on the one hand and amyloid beta
(1-42) and the formation of free radicals on the other
is not clear. Though there is a need for further data
about its role in the body the physiological role of tau
is well researched and it has been proved that it is a
compound able to fight wild oxidative and heat stress
and to take part in the maintenance of homeostasis
(Sultan et al. 2011). Brain tissue damage can cause
hypoxia and thus generate oxidative stress (Guzy et
al. 2005).
CURRENT OPTIONS FOR ALZHEIMER'S
DISEASE TREATMENT
The pharmacotherapy of AD is based on the
moderation of its manifestation, and calming of
depressions, aggression etc. is a common process for
the treatment of AD as well as the other dementias.
Causative treatment of AD is not currently available.
The common AD therapies mainly use inhibition of
enzyme acetylcholinesterase (AChE), an enzyme
participating in the termination of cholinergic
neurotransmission. As the lack of the neurotransmitter
acetylcholine is a negative process in the brain of AD
patients, the AChE inhibitors can resolve
acetylcholine deprivation. There are many known
AChE inhibitors, but the prospects for AD treatment
are conditioned by good penetration through the
blood brain barrier. Inhibitors so far tested, such as
the tetrahydroacridinium derivative tacrine and
organophosphate metrifonate (trichlorfon) have
adverse effects and clinical application has been
terminated (Lopez-Arrieta and Schneider 2006,
Alfirevic et al. 2007). The derivatives of tacrine have
also been extensively investigated; however, they are
not approved for therapeutic purposes (Pohanka et al.
2008, 2009, Korabecny et al. 2010). The AChE
inhibitors currently available for AD treatment are
donepezil, rivastigmine, and galantamine (Bonner and
Peskind 2002). Another drug, huperzine, is being
considered for clinical application; its variant
huperzine A is especially preferred and acts not only
as a non-competitive AChE inhibitor, but also as a
non-competitive N-methyl-D-aspartate (NMDA)
receptor antagonist (Zhang and Hu 2001). Some
countries, such as China, provide huperzine as a
regular drug (Desilets et al. 2009).
Memantine is the only available AD drug that acts
in a way other than on the cholinergic system. It is a
non-competitive antagonist of the NMDA receptor
binding in the open channel form of the receptor
(Potter 2010), which is a non selective ion channel
playing an excitatory role. It is speculated that the
receptor is involved in Alzheimer's disease aaetiology
as it enhances the deposition of amyloid oligomers
(Decker et al. 2010). Memantine is implicated in the
reduction of amyloid beta (1-42) toxicity and is
probably able to attenuate tau phosphorylation (Song
et al. 2008). It can also protect neurons from calcium
induced excitotoxicity (Lipton 2005). However,
despite being well suited for the amelioration of some
negative processes, it is not able either to resolve or to
significantly slow down the progression of AD
(Bassil et al. 2010). Past and current drugs for AD
treatment are summarized in Table 1.
It has been proposed that other pathways can be
affected in AD treatment. Inhibitors of gamma and beta
secretases are promising compounds for clinical trials
and growing interest can be expected in the
development of novel compounds influencing
secretases activity (He et al. 2010). As AD might be
a consequence of oxidative insult and/or activation of
the glial cells, the next effort will probably be aimed
at resolving inflammatory processes and oxidative
stress in the early stages of AD. From this point of
view, melatonin can be a prospective compound in
several pathways.
ACTION OF ANTIOXIDANTS IN
PATHOGENESIS OF ALZHEIMER'S
DISEASE
As the pathogenesis of AD is strongly related to the
generation of oxygen and nitrogen reactive species,
the application of low molecular weight antioxidants
can be hypothesized as suitable for its counteraction.
There is for example a proven protective effect of
vitamin E on neural cell culture exposed to 42 amino
acids long amyloid beta (Yatin et al. 2000) and
similar results have been noted in animal brains
(Butterfield 2002). However, plausible confirmation
of vitamin E or any other low molecular weight
antioxidant protective or therapeutic effect is missing,
despite extensive investigation. Devore et al. (2010) disclaimed any plausible beneficial effect of vitamin
C, beta carotene, and flavonoids on the onset of AD
pathogenesis. On the other hand, they found slight
improvement in AD as an effect of vitamin E when
administered in high doses. Most of the experimental
work unfortunately, has failed to follow the
composition of the vitamin E supplement. As seen
from the work of Mangialasche et al. (2010), for
example, the forms of vitamin E have unequal
efficacy. In vitamin E preparation, beta tocopherol had
better efficacy than alpha tocopherol, alpha tocotrienol, and beta
tocotrienol. Clinical trials proved a slight
improvement in AD progression due to tocopherols,
but the effect is not protective enough (Sano et al.
1997). Moreover, the low effect of the treatment
process can be overbalanced by the adverse effects of
antioxidants (Soni et al. 2010). It should also be
emphasized that the previously noted improvements
are only slight and the effects are hard to recognize.
Co-application of vitamin E with AD drugs is,
nevertheless, recommended in AD therapy
(Doraiswamy 2002).
Table 1. Past and current drugs for Alzheimer's disease treatment.
| Drug |
Systematic name |
Mechanism of action |
Fate | | Donepezil |
(RS)-2-[(1-benzyl-4-piperidyl)methyl]-5,6-dimethoxy-2,3-
dihydroinden-1-one |
non-competitive (reversible)
AChE inhibitor |
Marketed under trade name
Aricept | | Rivastigmine |
(S)-N-Ethyl-N-methyl-3-[1-
(dimethylamino)ethyl]phenyl
carbamate |
pseudoirreverzible inhibition of
AChE and BChE |
Available under trade name
Exelon | | Galantamine |
(4aS,6R,8aS)-5,6,9,10,11,12-
hexahydro-3-methoxy-11-
methyl-4aH-[1]benzofuro[3a,3,2-ef] [2] benzazepin-6-ol |
competitive (reversible)
inhibition of AChE |
Marketed under different trade
names such as Nivalin,
Razadyne, Reminyl... | | Huperzine A |
(1R,9S,13E)-1-Amino-13-
ethylidene-11-methyl-6-
azatricyclo[7.3.1.02,7] trideca-
2(7),3,10-trien-5-one |
non-competitive (reversible)
inhibitor of AChE and
non-competitive antagonist of
NMDA receptor |
Available in China; clinical
trials are not concluded | | Metrifonate |
(RS)-2,2,2-trichloro-1-
dimethoxyphosphoryl-ethanol |
irreversible inhibitor of AChE
and BChE |
Withdrawn in AD treatment due
to adverse effects; available as
antihelmintic | | Tacrine |
1,2,3,4-tetrahydroacridin-9-
amine |
non-competitive (reversible)
inhibitor of AChE and BChE |
Originally marketed as Cognex;
withdrawn due to adverse
effects, especially
hepatotoxicity | | Memantine |
3,5-dimethyladamantan-1-
amine |
non-competitive antagonist of
NMDA receptor binding in the
open channel form of receptor |
Marketed under trade names
Abixa, Axura, Ebixa, Memox,
Namenda... |
AChE - acetylcholinesterase; BChE - butyrylcholinesterase; NMDA - N-methyl-D-aspartic acid
MELATONIN BIOLOGICAL EFFECTS
Melatonin (N-acetyl-5-methoxytryptamine) is a pineal
gland hormone regulating time cyclicity in both
animals and humans (Prendergast 2010). In the lower
life forms, melatonin can act as an antioxidant
protecting against the harmful impact of reactive
oxygen and nitrogen species (Bustos-Obregon et al.
2005). In mammals, two G-coupled melatonin
receptors are known: melatonin receptors MT1 and
MT2. The receptors are responsible for circadian and
seasonal responses, but the physiological implications
are not fully recognized (Sugden et al. 2004). It has
also become plausible that circadian regulation and
hypnotic action are completely separate processes in
the action of melatonin (Jan et al. 2011b). Beside the
melatonin receptor, melatonin can act as an agonizing
ligand of the retinoic acid receptor-related orphan
receptor (ROR) alpha1 with the potential to control cell
cycle and apoptosis-associated genes (Wiesenberg et
al. 1995, Hill et al. 2009). The proven molecular
impacts of melatonin are summarized in Fig. 1.
The role of melatonin as an endogenous
antioxidant in humans and vertebrates is not clearly
understood. Moreover, the lack of melatonin could be
the reason for the higher incidence of Parkinson's
disease and cancer in night workers and some other
specific occupations (Schernhammer et al. 2006). In
recent years, melatonin has been considered as a drug
suitable for the suppression of the toxic impact of
many compounds and ameliorating the pathogenesis
of some diseases because of its fast suppression of
oxidative stress (Korkmaz et al. 2009). Another
impact - based on its expression of the IL-2 receptor
- is not clearly understood. In animal models, it has
been proved [by, for example, Mollace et al. (2005)]
that melatonin mediates the inhibition of inducible
nitric oxide synthase (iNOS), and cyclooxygenase 2
(COX2), and elevated levels of superoxide dismutase,
glutathione reductase and glutathione peroxidase (as
noted by, for example, Winiarska et al. 2006,
Venkataraman et al. 2010). Melatonin could act as a
very strong antioxidant. In comparison with the
typical endogenous antioxidants, melatonin is an
irreversible (also called 'suicidal') low molecular
weight antioxidant. This means that the oxidized form
of melatonin does not act as a pro-oxidative agent
deteriorating redox status in other tissues, as is typical
for the other antioxidants, and that it is not recovered
into its initial molecule by the simple redox system.
Moreover, the melatonin consumption products
6-hydroxymelatonin and N-acetyl-N-formyl-5-
methoxykynurenamine, also act as antioxidants
(Maharaj et al. 2007). The structures of melatonin and
its degradation products are summarized in Fig. 2.
The antioxidative effect of melatonin was recognized
as suitable for its performance as a non-specific
antioxidant after exposure to, for example, sulfur
mustard (Pohanka et al. 2011a) or methamphetamine
(Nopparat et al. 2010). On the other hand, there is
some controversy in work on the impact of melatonin
as the pro-oxidant activities of melatonin have also
been recognized in human erythrocytal proteins
(Dikmernoglu et al. 2008).
MELATONIN POTENTIAL FOR
ALZHEIMER'S DISEASE TREATMENT
The prospects for melatonin as a treatment for AD are
based on two independent pathways: a) scavenging of
reactive oxygen and nitrogen species, and b) acting as
a hormone or resolving sleep disturbance. Regarding
the antioxidant action, oxidized melatonin does not
act as a pro-oxidative agent, unlike the other
endogenous low molecular weight antioxidants such
as vitamin C, vitamin E, and glutathione (Tan et al.
2000). For this reason, melatonin could be found
suitable in AD treatment in situations where the other
antioxidants fail. On the other hand, rats with a
melatonin enriched diet have been found to have
significantly decreased levels of the antioxidant
homocysteine (Murawska-Cialowicz et al. 2008).
This points to a quite complex role for melatonin in
organisms that could be negative as well as positive
unless the application is supported by plausible
experimentation. The prospects for melatonin in
neuropathological processes in AD patients are
underlined by the fact that the levels of melatonin can
be low, as proved by Zhou et al. (2003) in an
experiment on 121 subjects investigated postmortem.
They recognized that individuals with a higher
physiological level of melatonin had a reduced level
of amyloid plaques and neurofibrillary tangles.
The role of melatonin as a potent molecule for the
suppression of oxidative stress has been investigated
by many teams. Experiments have shown that
melatonin can ameliorate the overproduction of
reactive oxygen species generated by complex I of the
mitochondrial respiration chain by way of cardiolipin,
an important part of the mitochondrial inner
membrane protection (Petrosillo et al. 2008).
Melatonin can keep the mitochondrial membrane
fluidity as it protects from lipid peroxidation and it is
speculated that mitochondrial membrane protection
can slow down age related degeneration (Garcia et al.
1997, 2010). However, this hypothesis needs to be
confirmed and applied particularly to AD
development in the early phases of pathogenesis.
Recently, it was proved that the processes of
senescence are altered in melatonin treated animals.
Melatonin significantly modified not only oxidative
stress related impairments, but also apoptotic
processes and macroautophagic activities in
senescence accelerated and slowed mice (Caballero et
al. 2009). Alterations in NFkappaB, iNOS, TNFalpha and IL1
levels in a mice model are also involved in the
biological effect of melatonin (Cuesta et al. 2010).
Considering that AD, Parkinson's disease and some
other neurodegenerative disorders are thought to be
related to chronic inflammation (Gemma 2010), melatonin could be regarded as a compound for
inflammation control (Wang and Wang 2006). On the
other hand, if neurodegeneration is caused by
neuroinflammation alone, the application of a
standard steroidal or non-steroidal anti-inflammatory
drug would be preferable. The role of the immune
system in the nervous system is a complex one, and it
can be detrimental when the autoimmune response for
chronic inflammation is launched. Besides, the
immune system function is necessary for protection
against an invasion of pathogens (Gendelman 2002).
It can be questioned whether the application of
melatonin increases sensitivity to pathogens and
enables pathogen invasion when the above mentioned
suppression of innate immunity is considered.
Unfortunately, this question has not yet been fully
solved.
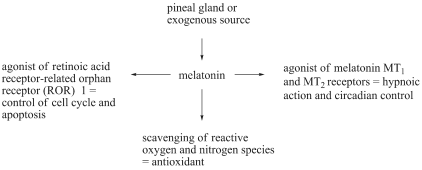
Fig. 1. Summarization of melatonin impact in mammal organism.
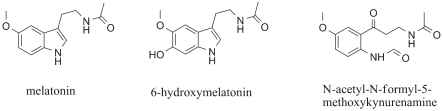
Fig. 2. Structures of melatonin and the most significant products of its oxidative degradation.
The link between circadian timing and the
immune system has been hypothesized by many
scientists (Berger 2008). The implication of melatonin
in the modulation of apoptosis is similar to the effect
of another antioxidant, epigallocatechin gallate. This
green tea antioxidant was found to be able to
influence extensively apoptosis in healthy mice
exposed to sulfur mustard (Pohanka et al. 2011b).
The application of 10 mg/kg melatonin was found
to be suitable for the suppression of some detrimental
processes in the nervous system. It also triggered the
up-regulation of the ROR alpha1 level. On the other hand,
the application was without any implication in MT1
receptor presence in SAMP8 senescence accelerated
mice and senescence slowed mice SAMR1 (Caballero
et al. 2008). In this mouse model, the effects are
corroborated for a long term application (five or ten
months) that represents half and more of the life term
given normal life expectancy. As reported by
Gutierrez-Cuesta et al. (2007), the chronic admini-stration of melatonin 10 mg/kg also had another
significant effect in SAMP8 mice: reduced cell loss
and oxidative damage of macromolecules. They
found not only decreased hyperphosphorylation of
tau, but also down-regulated activation of cdk5/p35
and its cleavage to cdk5/p25; these point to a link
between melatonin and reduced neurodegeneration on
the level of its molecular control. On the other hand,
it should be emphasized that the effect described
followed quite a high dose of melatonin. Were the
dose to be recalculated to the average human weight,
it would be approximately two hundred times higher
than the dose 3 mg pro toto used for improvement of
sleep quality (Nunes et al. 2008).
Melatonin action as a hormone can be suitable for
the treatment of sleep disturbances. AD patients have
fragmented sleep, disturbing the normal sleep-wake
circadian rhythm (Song et al. 2010); melatonin can
correct this and improve physical as well as mental
shape (David et al. 2010). It should also be noted that
melatonin production decreases with age and that it is
produced on a limited scale in AD patients so that the
administration could substitute for the suppressed
function of the pineal gland. This reduced production
is considered a possible cause of the beneficial effect
of melatonin pharmacological performance (Karasek
and Reiter 2002, Karasek 2004). Although melatonin
can influence the body in several ways and is a
compound of considerable scientific interest, most of
the results discussed were found in animal models or
cell lines. Any clinically confirmed melatonin
beneficial effects should be further investigated in a
wide study as some of the preliminary results are
encouraging: in particular its efficacy in the
improvement of sleep in patients should be critically
assessed. In considering any clinically proved
efficacy of melatonin to treat insomnia in the elderly
(Wade et al. 2010), it should again be noted that
beneficial physical effects in humans are strongly
influenced by sleep improvement alone. Also, Furio
et al. (2007) have ascribed the effect of melatonin in
a AD related study to circadian regulation rather than
an antioxidant action. Similar conclusions were
reached by Dowling et al. (2008). However, their
study was carried out on a total of fifty subjects
treated for ten weeks. The groups of 16, 17 and 17
specimens are quite small and smaller changes were
not probably recognized. A systematic review carried
out in 2005 provided similar results: there is
insufficient evidence to support the effectiveness of
melatonin in the treatment of the cognitive and
non-cognitive sequences of dementia (Jansen et al.
2006). More responsible data can be observed when
melatonin is administered to people 55 years and over
together with the investigation of the occurrence of
new AD cases and the further progression of AD.
Unfortunately, such clinical trials have not yet taken
place.
CONCLUSIONS AND EXPECTATIONS FOR
THE FUTURE
Despite the significant effect of melatonin on
neuro-degeneration in experimental models, its
potency in protecting neurons from aggravation is
neither fully understood nor plausibly recognized in
clinical studies. For the next decades, experiments
and trials on human beings can be expected. The
pertinent experiments based on application of
melatonin to human beings suffering from
neurodegenerative disorders are now simplified by
the fact that melatonin has been approved as a food
supplement and drug for insomnia by the (U.S.) Food
and Drug Administration agency and there are only
minimal or no adverse effects (Taylor and Weiss
2009). The favourability of melatonin for treatment is
emphasized by two facts: melatonin is quite cheap
and simple to synthesis as a derivative of tryptophan
with an intact indol core (Estevao et al. 2010) and
melatonin is maintained in the body for a long time.
Some authors [e.g. Mistraletti et al. (2010)] have
referred to the keeping of the pharmacological level
in serum for 10 hours following enteral administration
when the pharmacological level is reached after
approximately 5 minutes with serum peak after 16
minutes.
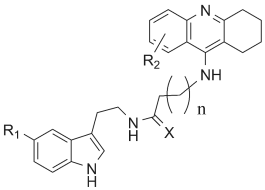
Fig. 3. Derivatives composed from tacrine and
melatonin prepared by Fernandez-Bachiller et al. (2009).
R1=-H, -OCH3; R2=-H, -Cl, bis Cl (6,8 position); X=O or S;
n=4, 5, 6, or 7.
Recent investigations have proposed that the
deposition of amyloid plaque may be accelerated or
even triggered by the interaction of amyloid beta
(1-42) with the anionic subsite of AChE (Castro and
Martinez 2006). Considering the stress insult
necessary for amyloid beta deposition and amyloid
plaque, the conjugates of the AChE inhibitor with
antioxidant are promising drugs of the next
generation. Tacrine-melatonin heterodimers have
been extensively investigated in addition to the other
compounds (Tumiatti et al. 2010). We can speculate
how prepared compounds can be effective not only
for the amelioration of AD symptomatic
manifestation but also because of their ability to slow
down the pathogenesis progression. For example
Fernandez-Bachiller et al. (2009) prepared derivatives
of tacrine linked to melatonin via amine of tacrine and
N-acetyl of melatonin. The prepared derivatives (see
common structure in Fig. 3) kept good inhibitory
potency to AChE as well as antioxidant ability in
vitro. Especially the chloro and bischloro derivates of
tacrine linked to melatonin by six carbon long chains,
had significantly higher selectivity to AChE
compared to butyrylcholinesterase (BChE) retaining
antioxidant ability and strong inhibitory potency to
human AChE. We can expect next an effort to
prepare new melatonin derivatives and confirmation
of their effects on animal models in the short or
medium term range. Unfortunately, the pertinent
performance of these novel derivatives is limited, as
the biological effect of melatonin complex on
neurodegeneration is not fully understood.
ACKNOWLEDGEMENT
The Czech Science Foundation is gratefully
acknowledged for the project No. P303/11/1907.
REFERENCES
Alfirevic A, Mills T, Carr D, Barratt BJ, Jawaid A, Sherwood J, Smith JC, Tugwood J, Hartkoorn R, Owen A, Park KB, Pirmohamed M. Tacrine-induced liver damage: an analysis of 19 candidate genes. Pharmacogenet Genomics. 17: 1091-1100, 2007.
[CrossRef]
Bassil N, Thaipisuttikul P, Grossberg GT. Memantine ER, a one-daily formulation for the treatment of Alzheimer’s disease. Expert Opin Pharmacother. 11: 1765-1771, 2010.
[CrossRef]
Berger J. A two-clock model of circadian timing in the immune system of mammals. Pathol Biol (Paris). 56: 286-291, 2008.
[CrossRef]
Bonner LT, Peskind ER. Pharmacologic treatments of dementia. Med Clin North Am. 86: 657-674, 2002.
Boyd-Kimball D, Sultana R, Mohammad-Abdul H, Butterfield DA. Rodent Abeta(1-42) exhibits oxidative stress properties similar to those of human Abeta(1-42): Implication for proposed mechanisms of toxicity. J Alzheimers Dis. 6: 515-525, 2004.
Bustos-Obregon E, Gonzalez JR, Espinoza O. Melatonin as protective agent for the cytotoxic effects of diazinon in the spermatogenesis in the earthworm Eisenia foetida. Ital J Anat Embryol. 110: 159-165, 2005.
Butterfield DA. Amyloid beta-peptide (1-42)-induced oxidative stress and neurotoxicity: implications for neurodegeneration in Alzheimer’s disease brain. A review. Free Radic Res. 36: 1307-1313, 2002.
Butterfield DA, Kanski J. Methionine residue 35 is critical for the oxidative stress and neurotoxic properties of Alzeheimer’s amyloid beta-peptide 1-42. Peptides. 23: 1299-1309, 2002.
Butterfiedl DA, Lauderback CM. Lipid peroxidation an protein oxidation in Alzheimer’s disease brain: potential causes and consequences involving amyloid beta-peptide-associated free radical oxidative stress. Free Radic Biol Med. 32: 1050-1060, 2002.
[CrossRef]
Caballero B, Vega-Naredo I, Sierra V, Huidobro-Fernandez C, Soria-Valles C, De Gonzalo-Calvo D, Tolivia D, Gutierrez-Cuesta J, Pallas M, Camins A, Rodriguez-Colunga MJ, Coto-Montes A. Favorable effects of a prolonged treatment with melatonin on the level of oxidative damage and neurodegeneration in sensescen-ce-accelerated mice. J Pineal Res. 45: 302-311, 2008.
[CrossRef]
Caballero B, Vega-Naredo I, Sierra V, Huidobro-Fernandez C, Sorie-Valles C, De Gonzalo-Calvo D, Tolivia D, Pallas M, Camins A, Rodriguez-Colunga MJ, Coto-Montes A. Melatonin alters cell death processes in response to age-related oxidative stress in the brain of senescence-accelerated mice. J Pineal Res. 46: 106-114, 2009.
Cai T, Che H, Yao T, Chen Y, Huang C, Zhang W, Du K, Zhang J, Cao Y, Chen J, Luo W. Manganese induces tau hyperphosphorylation through the activation of ERK MAPK pathway in PC12 cells. Toxicol Sci. 119: 169-177, 2011.
[CrossRef]
Casoli T, Di Stefano G, Balietti M, Solazzi M, Giorgetti B, Fattoretti P. Peripheral inflammatory biomarkers of Alzheimer’s disease: the role of platelets. Biogerontology. 11: 627-633, 2010.
[CrossRef]
Castro A, Martinez A. Targeting beta-amyloid pathogenesis through acetylcholinesterase inhibitors. Curr Pharm Des. 12: 4377-4387, 2006.
Clementi ME, Misiti F. Substitution of methionine 35 inhibits apoptotic effects of Abeta(31-35) and Abeta (25-35) fragments of amyloid beta protein in PC12 cells. Med Sci Monit. 11: BR381-385, 2005.
Clementi ME, Pezzotti M, Orsini F, Sampaolese B, Mezzogori D, Grassi C, Giardina B, Misiti F. Alzheimer’s amyloid beta-peptide (1-42) induces cell death in human neuroblastoma via bax/bcl-2 ratio increase: an intriguing role for methionine 35. Biochem Biophys Res Commun. 342: 206-213, 2006.
[CrossRef]
Coen K, Annaert W. Presenilins: how much more than gamma-secretase?! Biochem Soc Trans. 38: 1474-1478, 2010.
[CrossRef]
Cole SL, Vassar R. The Alzheimer’s disease beta-secretase enzyme, BACE I. Mol Neurodeg. 2: 22, 2007.
[CrossRef]
Cuesta S, Kireev R, Forman K, Garcia C, Escames G, Ariznavarreta C, Vara E, Tresguerres JAF. Melatonin improves inflammation processes in liver of senescence-accelerated prone male mice (SAMP8). Exp Gerontol. 45: 950-956, 2010.
[CrossRef]
David R, Zeitzer J, Friedman L, Noda A, O’Hara R, Robert P, Yesavage JA. Non-pharmacologic management of sleep disturbance in Alzheimer’s disease. J Nutr Health Aging. 14: 203-206, 2010.
Decker H, Jurgensen S, Adrover MF, Brito-Moreira J, Bomfim TR, Klein WL, Epstain AL, De Felice FG, Jerusalinsky D, Ferreira ST. N-methyl-D- aspartate receptors are required for synaptic targeting of Alzheimer’s toxic amyloid-beta peptide oligomers. J Neurochem. 115: 1520-1529, 2010.
[CrossRef]
Desilets AR, Gickas JJ, Dunican KC. Role of huperzine A in the treatment of Alzheimer’s disease. Ann Pharmacother. 43: 514-518, 2009.
[CrossRef]
Devore EE, Grodstein F, van Rooij FJ, Hofman A, Stampfer MJ, Witteman JC, Breteler MM. Dietary antioxidants and long-term risk of dementia. Arch Neurol. 67: 819-825, 2010.
[CrossRef]
Dikmernoglu N, Ileri E, Seringec N, Ercil D. Melatonin prevents lipid peroxidation in human erythrocytes but augments deterioration of deformability after in vitro oxidative stress. Clin Hemorheol Microcirc. 40: 235-242, 2008.
[CrossRef]
Doraiswamy PM. Non-cholinergic strategies for treating and preventing Alzheimer’s disease. CNS Drugs. 16: 811-824, 2002.
Dowling GA, Burr RL, Van Someren EJ, Hubbard EM, Luxenberg JS, Mastick J, Cooper BA. Melatonin and bright-light treatment for rest-activity disruption in institutionalized patients with Alzheimer’s disease. J Am Geriatr Soc. 56: 239-246, 2008.
[CrossRef]
Estevao MS, Carvalho LC, Ribeiro D, Couto D, Freitas M, Gomes A, Ferreira LM, Fernandes E, Marques MMB. Antioxidant activity of unexplored indole derivatives: synthesis and screening. Eur J Med Chem. 45: 4869-4878, 2010.
[CrossRef]
Fernandez-Bachiller MI, Perez C, Campillo NE, Paez JA, Gonzalez-Munoz GC, Usan P, Garcia-Palomero E, Lopez MG, Villaroya M, Garcia AG, Martinez A, Rodriguez-Franco MI. Tacrine- melatonin hybrids as multifunctional agents for Alzheimer’s disease, with cholinergic, antioxidant, and neuroprotective properties. Chem Med Chem. 4: 828-841, 2009.
[CrossRef]
Flaherty DP, Kiyota T, Dong Y, Ikezu T, Vennerstrom JL. Phenolic bis-styrylbenzenes as beta-amyloid binding ligands and free radicals scavengers. J Med Chem. 53: 7992-7999, 2010.
[CrossRef]
Furio AM, Brusco LI, Cardinali DP. Possible therapeutic value of melatonin in mild cognitive impairement: a retrospective study. J Pineal Res. 43: 404-409, 2007.
[CrossRef]
Garcia JJ, Reiter RJ, Guerrero JM, Escames G, Yu BP, Ho CS, Munoz-Hoyos A. Melatonin prevents changes in microsomal membrane fluidity during induced lipid peroxidation. FEBS Lett. 408: 297-300, 1997.
Garcia JJ, Pinol-Ripoll G, Martinez-Ballarin E, Fuentes-Broto L, Miana-Mena FJ, Venegas C, Caballero B, Escames G, Coto-Montes A, Acuna-Castroviejo D. Melatonin reduces membrane rigidity and oxidative damage in the brain of SAMP(8) mice. Neurobiol Aging, 2010 - In press, doi: 10.1016/j.neurobiolaging. 2009.12.013.
Gemma C. Neuroimmunomodulation and aging. Aging Dis. 1: 169-172, 2010.
Gendelman HE. Neural immunity: friend or foe? J Neurovirol. 8: 474-479, 2002.
[CrossRef]
Gilca M, Stoian I, Atanasiu V, Virgolici B. The oxidative hypothesis of senescence. J Postgrad Med. 53: 207-213, 2007.
[CrossRef]
Gutierrez-Cuesta J, Sureda FX, Romeu M, Canudas AM, Caballero B, Coto-Montes A, Camins A, Pallas M. Chronic administration of melatonin reduces cerebral injury biomarkers in SAMP8. J Pineal Res. 42: 394-402, 2007.
[CrossRef]
Guzy RD, Hoyos B, Robin E, Chen H, Liu L, Mansfield KD, Simon MC, Hammerling U, Schumacker PT. Mitochondrial complex III is required for hypoxia-induced ROS production and cellular oxygen sensing. Cell Metab. 1: 401-408, 2005.
[CrossRef]
He G, Luo W, Li P, Remmers C, Netzer WJ, Hendrick J, Bettayeb K, Flajolet M, Gorelick F, Wennogle LP, Greengard P. Gamma-secretase activating preotein is a therapeutic target for Alzheimer’s disease. Nature. 467: 95-98, 2010.
[CrossRef]
Hill SM, Frasch T, Xiang S, Yuan L, Duplessis T, Mao L. Molecular mechanisms of melatonin anticancer effects. Integr Cancer Ther. 8: 337-346, 2009.
Ill-Raga G, Ramos-Fernandez E, Guix FX, Tajex M, Bosch-Morato M, Palomer E, Godoy J, Belmar S, Cerpa W, Simpkins JW, Inestrosa And NC, Munoz FJ. Amyloid-beta peptide fibrils induce nitro-oxidative stress in neuronal cells. J Alzheimers Dis. 22: 641-652, 2010.
[CrossRef]
Jan A, Adolfosson O, Allaman I, Buccarello AL, Magistretti PJ, Pfeifer A, Muhs A, Lashuel HA. A(beta)42 neurotoxicity is mediated by ongoing nucleated polymerization process rather than by discrete A(beta)42 species. J Biol Chem. 286: 8585-8596, 2011a.
[CrossRef]
Jan JE, Reiter RJ, Wong PK, Bax MC, Ribary U, Wasdell MB. Melatonin has membrane receptro-independent hypnotic action on neurons: an hypothesis. J Pineal Res. 50: 233-240, 2011b.
[CrossRef]
Jansen SL, Forbes DA, Morgan DG. Melatonin for cognitive impairment. Cochrane Database Syst Rev. CD003802.pub3, 2006.
[CrossRef]
Jonsson L, Wimo A. The cost of dementia in Europe: a review of the evidence, and methodological considerations. Pharmacoeconomics. 27: 391-403, 2009.
[CrossRef]
Juranek I, Bezek S. Controversy of free radical hypothesis: reactive oxygen species - cause or consequence of tissue injury? Gen Physiol Biophys. 24: 263-278, 2005.
Kadlcik V, Sicard-Roselli C, Mattioli TA, Kodicek M, Houee-Levin C. One-electron oxidation of beta-amyloid peptide: sequence modulation of reactivity. Free Radic Biol Med. 37: 881-891, 2004.
[CrossRef]
Kao PF, Davis DA, BAnigan MG, Vanderburg CR, Seshadri S, Delalle I. Modulators of cytoskeletal rearganization in CA1 hippocampal neurons show increased expression in patients at mid-stage Alzheimer’s disease. PLoS One. 5:e13337, 2010.
[CrossRef]
Karasek M. Melatonin, human aging, and age-related diseases. Exp Gerontol. 39: 1723-1729, 2004.
[CrossRef]
Karasek M, Reiter RJ. Melatonin and aging. Neuro Endocrinol Lett. 1: 14-16, 2002.
Korabecny J, Musilek K, Holas O, Binder J, Zemek F, Marek J, Pohanka M, Opletalova V, Dohnal V, Kuca K. Synthesis and in vitro evaluation of N-alkyl-7-methoxytacrine hydrochlorides as potential cholinesterase inhibitors in Alzheimer disease. Bioorg Med Chem Lett. 20: 6093-6095, 2010.
[CrossRef]
Korkmaz A, Reiter RJ, Topal T, Manchester LC, Oter S, Tan DX. Melatonin: an established antioxidant worthy of use in clinical trials. Mol Med. 15: 43-50, 2009.
Kuperstein I, Broersen K, Benilova I, Rozenski J, Jonckheere W, Debulpaep M, Vandersteen A, Sagers-Nolten I, Van Der Werf K, Subramaniam V, Braeken D, Callewaert G et al. Neurotoxicity of Alzheimer’s disease Abeta peptides is induced by small changes in the A(beta)42 to A(beta)40 ratio. EMBO J. 29: 3408-3420, 2010.
[CrossRef]
Lei P, Ayton S, Finkelstein DI, Adlard PA, Masters CL, Bush AI. Tau protein: relevance to Parkinson’s disease. Int J Biochem Cell Biol. 42: 1775-1778, 2010.
[CrossRef]
Leinonen V, Koivisto AM, Savolainen S, Rummukainen J, Tamminen JN, Tillgren T, VAinikka S, Pyykko OT, Molsa J, Fraunberg M, Pirtilla T, Jaaskelainen JE et al. Amyloid and tau proteins in cortical brain biopsy and Alzheimer’s disease. Ann Neurol. 68: 446-453, 2010.
[CrossRef]
Lipton SA. The molecular basis of memantine action in Alzheimer’s disease and other neurologic disorders: low affinity, uncompetitive antagonism. Curr Alzheimer Res. 2: 155-165, 2005.
Lopez-Arrieta JM, Schneider L. Metrifonate for Alzheimer’s disease. Cochrane Database Syst Rev CD003155, 2006.
[CrossRef]
Maharaj DS, Glass BD, Daya S. Melatonin: new places in therapy. Biosci Rep. 27: 299-320, 2007.
[CrossRef]
Mangialasche F, Kivipelto M, Mecocci P, Rizzuto D, Palmer K, Winblad B, Fratiglioni L. High plasma levels of vitamin E forms and reduced Alzheimer’s disease risk in advanced age. J Alzheimers Dis. 20: 1029-1037, 2010.
[CrossRef]
Mistralleti G, Sabbatini G, Taverna M, Figini MA, Umbrello M, Magni P, Ruscica M, Dozio E, Esposti R, DeMartini G, Fraschini F, Rezzani R et al. Pharmacokinetics of orally administered melatonin in critically ill patients. J Pineal Res. 48: 142-147, 2010.
[CrossRef]
Mollace V, Muscoli C, Masini E, Cuzzocrea S, Salvemini D. Modulation of prostaglandin biosynthesis by nitric oxide and nitric oxide donors. Pharmacol Rev. 57: 217-252, 2005.
[CrossRef]
Murawska-Cialowicz E, Januszewska L, Zuwala-Jagiello J, Milczarska J, Zawadzki M, Paprocka-Borowicz M, Wierbicka-Damska I. Melatonin decreases homocysteine level in blood of rats. J Physiol Pharmacol. 59: 717-729, 2008.
Murphy MP, LeVine H. Alzheimer’s disease and the beta-amyloid peptide. J Alzheimers Dis. 19: 311-323, 2010.
[CrossRef]
Nopparat C, Porter JE, Ebadi M, Govitrapong P. The mechanism for the neuroprotective effect of melatonin against methamphetamine-induced autophagy. J Pineal Res. 49: 382-389, 2010.
[CrossRef]
Nunes DM, Mota RMS, Machado MO, Pereira EDB, de Bruin VMS, de Bruin PFC. Effect of melatonin administration on subjective sleep quality in chronic obstructive pulmonary disease. Braz J Med Biol Res. 41: 926-931, 2008.
Petrosillo G, Fattoretti P, Matera M, Ruggiero FM, Bertoni-Freddari C, Paradies G. Melatonin prevents age-related mitochondrial dysfunction in rat brain via cardiolipin protection. Rejuvenation Res. 11: 935-943, 2008.
[CrossRef]
Pickrell AM, Fukui H, Moraes CT. The role of cytochrome c oxidase deficiency in ROS and amyloid plaque formation. J Bioenerg Biomembr. 41: 453-456, 2009.
[CrossRef]
Pohanka M, Kuca K, Kassa J. New performance of biosensor technology for Alzheimer’s disease drugs: in vitro comparison of tacrine and 7-methoxytacrine. Neuroendocrinol Lett. 29: 755-758, 2008.
Pohanka M, Musilek K, Kuca K. Progress of biosensors based on cholinesterase inhibition. Curr Med Chem. 16: 1790-1798, 2009.
Pohanka M, Sobotka J, Jilkova M, Stetina R. Oxidative stress after sulfur mustard intoxication and its reduction by melatonin: efficacy of antioxidant therapy during serious intoxication. Drug Chem Toxicol. 34: 85-91, 2011a.
[CrossRef]
Pohanka M, Sobotka J, Stetina R. Sulfur mustard induced oxidative stress and its alteration by epigallocatechin gallate. Toxicol Lett., 2011b - In press, doi: 10.1016/j.toxlet.2010.12.011.
Potter PE. Investigational medications for treatment of patients with Alzheimer disease. J Am Osteopath Assoc. 110: S27-S36, 2010.
Prendergast BJ. MT1 melatonin receptors mediate somatic, behavioral, and reproductive neuroendocrine responses to photoperiod and melatonin in Siberian hamsters (Phodopus sungorus). Endocrinology. 15: 714-721, 2010.
[CrossRef]
Raji CA, Lopez OL, Kuller LH, Carmichael OT, Becker JT. Age, Alzheimer disease, and brain structure. Neurology. 73: 1899-1905, 2009.
[CrossRef]
Sano M, Enesto C, Thomas RG, Klauber MR, Schafer K, Grundman M, Woodbury P, Growdon J, Cotman CW, Pfeiffer E, Schneider LS, Thal LJ. A controlled trial of selegiline alpha-tocopherol, or both as treatment for Alzheimer’s disease. The Alzheimer’s Disease Cooperative Study. N Engl J Med. 336: 1216-1222, 1997.
[CrossRef]
Schernhammer E, Chen H, Ritz B. Circulating melatonin levels: possible link between Parkinson’s disease and cancer risk? Cancer Causes Control. 17: 577-582, 2006.
[CrossRef]
Schumock GT. Economic consideration in the treatment and management of Alzheimer’s disease. Am J Health Syst Pharm. 55: S17-S21, 1998.
Song MS, Rauw G, Baker GB, Kar S. Memantine protects rat cortical cultured neurons against beta-amyloid-induced toxicity by attenuating tau phosphorylation. Eur J Neurosci. 28: 1989-2002, 2008.
[CrossRef]
Song Y, Dowling GA, Wallhagen MI, Lee KA, Strawbridge WJ. Sleep in older aults with Alzheimer’s disease. J Neurosci Nurs. 42: 190-198, 2010.
Soni MG, Thurmond TS, Miller ER, Spriggs T, Bendich A, Omaye ST. Safety of vitamins and minerals: controversies and perspective. Toxicol Sci. 118: 348-355, 2010.
[CrossRef]
Sugden D, Davidson K, Hough KA, Teh MT. Melatonin, melatonin receptors and melanophores: a moving story. Pigment Cell Res. 17: 454-460, 2004.
[CrossRef]
Sultan A, Nesslany F, Violet M, Begard S, Loyens A, Talahari S, Mansuroglu Z, Marzin D, Sergeant N, Humez S, Colin M, Bonnefoy E, Buee L, Galas MC. Nuclear tau: a key player in neuronal DNA protection. J Biol Chem. 286: 4566-4575, 2011.
[CrossRef]
Tan DX, Manchester LC, Reiter RJ, Qi WB, Karbownik M, Calvo JR. Significance of melatonin in antioxidative defense system: reactions and products. Biol Signals Recept. 9: 137-159, 2000.
Taylor SR, Weiss JS. Review of insomnia pharmacotherapy options for the elderly: implications for managed care. Popul Health Manag. 12: 317-323, 2009.
[CrossRef]
Tumiatti V, Minarini A, Bolognesi ML, Milelli A, Rosini M, Melchiorre C. Tacrine derivatives and Alzheimer’s disease. Curr Med Chem. 17: 1825-1838, 2010.
Venkataraman P, Selvakumar K, Krishnamoorthy G, Muthusami S, Rameshkumar R, Prakash S, Arunakaran J. Effect of melatonin on PCB (Aroclor 1254) induced neuronal damage and changes in Cu/Zn superoxide dismutase and glutathione peroxidase-4 mRNA expression in cerebral cortes, cerebellum and hippocampus of adult rats. Neurosci Res. 66: 189-197, 2010.
[CrossRef]
Wade AG, Ford I, Crawford G, McConnachie A, Nir T, Laudon M, Zisapel N. Nightly treatment of primary insomnia with prolonged release melatonin for 6 months: a randomized placebo controlled trial on age and endogenous melatonin as predictors of efficacy and safety. BMC Med. 8: 51, 2010.
[CrossRef]
Wang JZ, Wang ZF. Role of melatonin in Alzheimer-like neurodegeneration. Acta Pharmacol Sin. 27: 41-49, 2006.
[CrossRef]
Wetzels RB, Zuidema SU, de Jonghe JF, Verhey FR, Koopmans RT. Course of neuropsychiatric symptoms in residents with dementia in nursing homes over 2-year period. Am J Geriatr Psychiatry. 18: 1054-1065, 2010.
Wiesenberg I, Missbach M, Kahlen JP, Schrader M, Carlberg C. Transcriptional activation of the nuclear receptor RZRalpha by the pineal gland hormone melatonin and identification of CGP 52608 as a synthetic ligand. Nucleic Acids Res. 23: 327-333, 1995.
Wilson RS, Mendes De Leon CF, Barnes LL, Schneider JA, Evans DA, Bennett DA. Participation in cognitively stimulating activities and risk of incident Alzheimer disease. JAMA. 287: 742-748, 2002.
Winiarska K, Fraczyk T, Malinska D, Drozak J, Bryla J. Melatonin attenuates diabetes-induced oxidative stress in rabbits. J Pineal Res. 40: 168-176, 2006.
[CrossRef]
Yatin SM, Varadarajan S, Butterfield DA. Vitamin E prevents Alzheimer’s amyloid beta-peptide (1-42) induced neuronal protein oxidation and reactive oxygen species production. J Alzheimers Dis. 2: 123-131, 2000.
Zhang JM, Hu GY. Huperzine A, a nootropic alkaloid, inhibits N-methyl-D-aspartate-induced current in rat dissociated hippocampal neurons. Neuroscience. 105: 663-669, 2001.
Zheng WH, Bastianetto S, Menniken F, Ma W, Kar S. Amyloid beta peptide induces tau phosphorylation and loss of cholinergic neurons in rat primary septal cultures. Neuroscience. 115: 201-211, 2002.
[CrossRef]
Zhou JN, Liu RY, Kamphorst W, Hofman MA, Swaab DF. Early neuropathological Alzheimer’s changes in aged individuals are accompanied by decreased cerebrospinal fluid melatonin levels. J Pineal Res. 35: 125-130, 2003.
|
BACK
|




